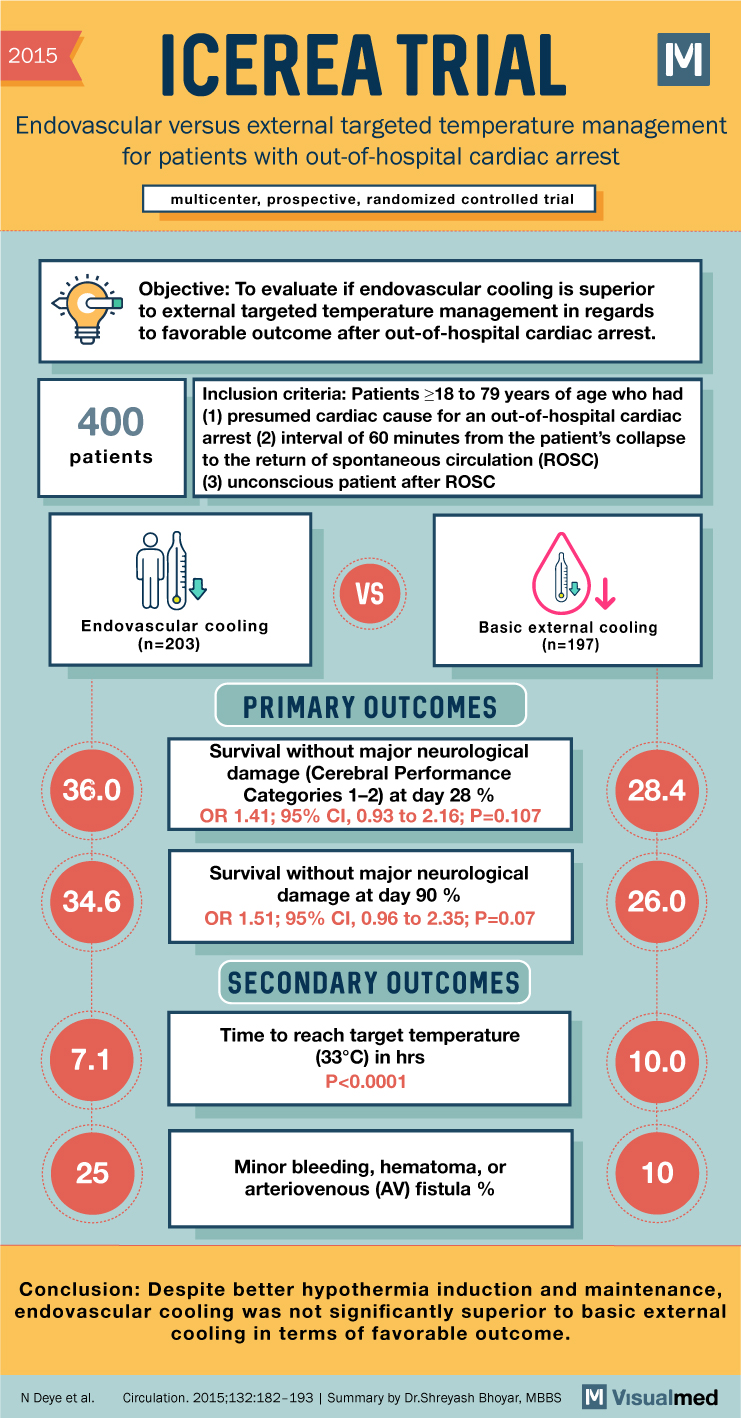
2015 ICEREA TRIAL Endovascular versus external targeted temperature management for patients with out-of-hospital cardiac arrest multicenter, prospective, randomized controlled trial Objective: To evaluate if endovascular cooling is superior to external targeted temperature management in regards to favorable outcome after out-of-hospital … Read More
Blog
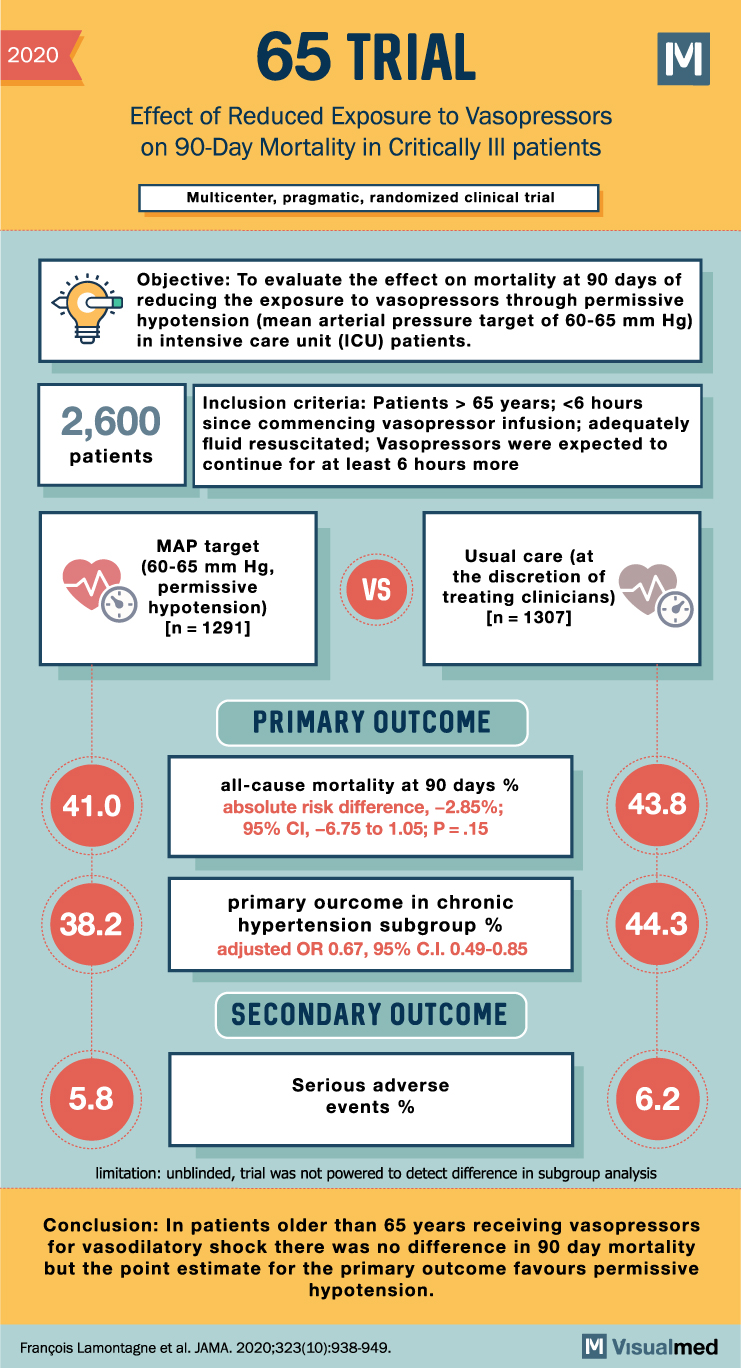
65 Trial Summary: Vasopressors in Older Critically Ill Patients
2020 65 TRIAL Effect of Reduced Exposure to Vasopressors on 90-Day Mortality in Critically III patients Multicenter, pragmatic, randomized clinical trial Objective: To evaluate the effect on mortality at 90 days of reducing the exposure to vasopressors through permissive hypotension … Read More
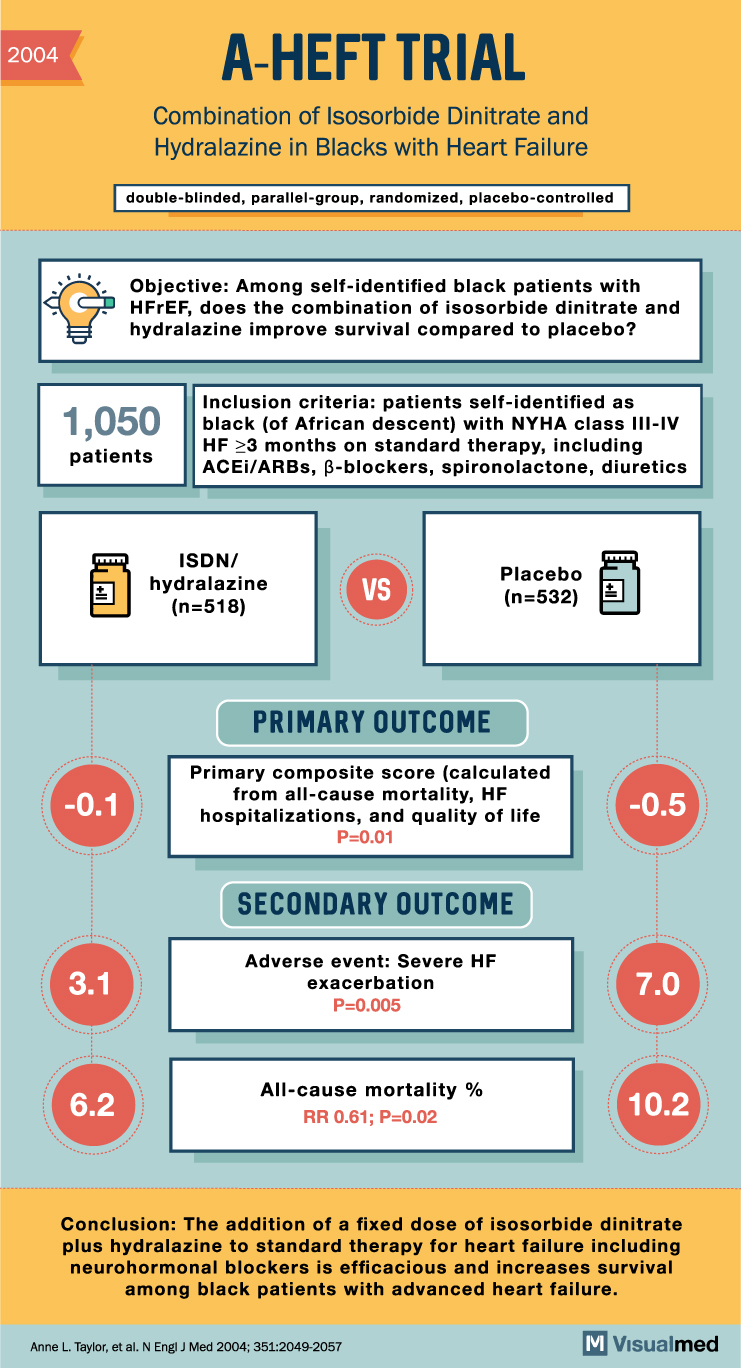
A-HEFT Trial Summary: Isosorbide Dinitrate and Hydralazine in Blacks with Heart Failure
2004 -0.1 3.1 A-HEFT TRIAL Combination of Isosorbide Dinitrate and Hydralazine in Blacks with Heart Failure Inclusion criteria: patients self-identified as 1,050 black (of African descent) with NYHA class III-IV patients HF ≥3 months on standard therapy, including ACEI/ARBs, ẞ-blockers, … Read More
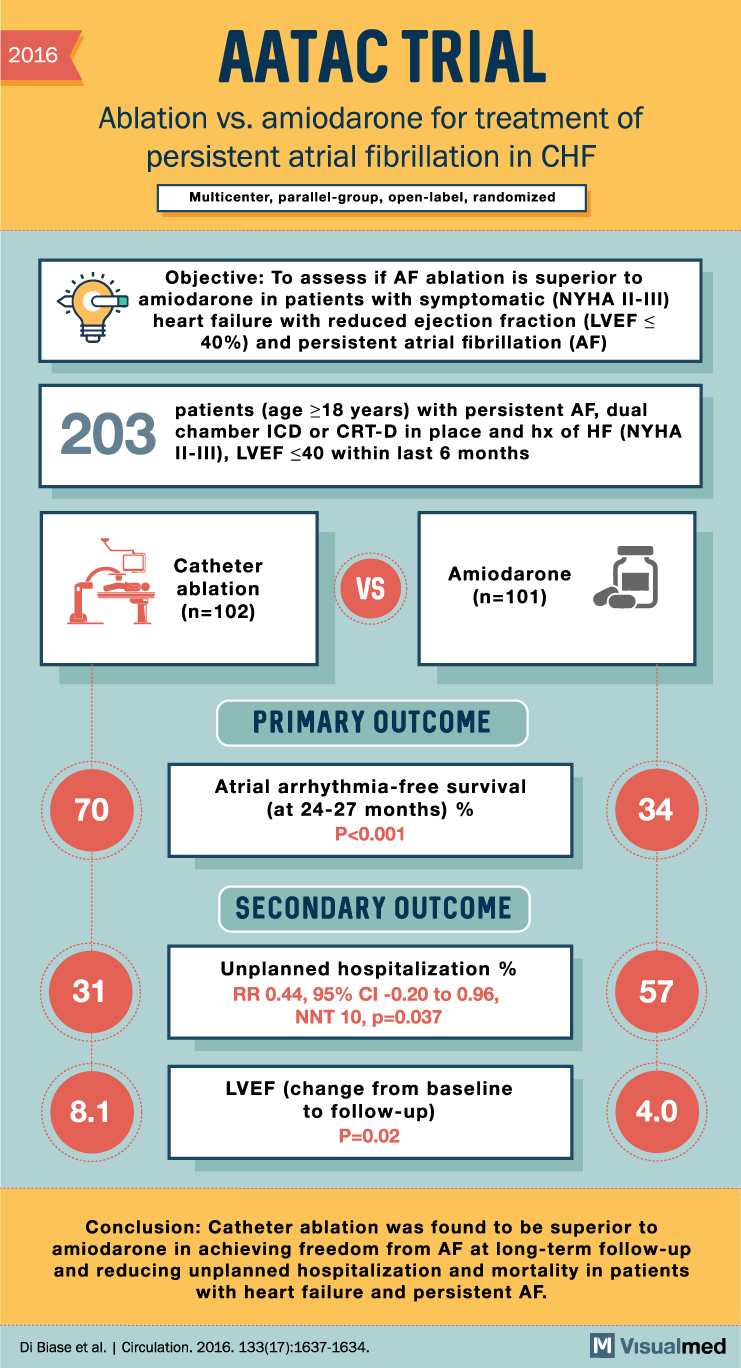
AATAC Trial Summary: Ablation vs. amiodarone for Persistent Atrial Fibrillation
2016 AATAC TRIAL Ablation vs. amiodarone for treatment of persistent atrial fibrillation in CHF Multicenter, parallel-group, open-label, randomized Objective: To assess if AF ablation is superior to y amiodarone in patients with symptomatic (NYHA II-III) heart failure with reduced ejection … Read More
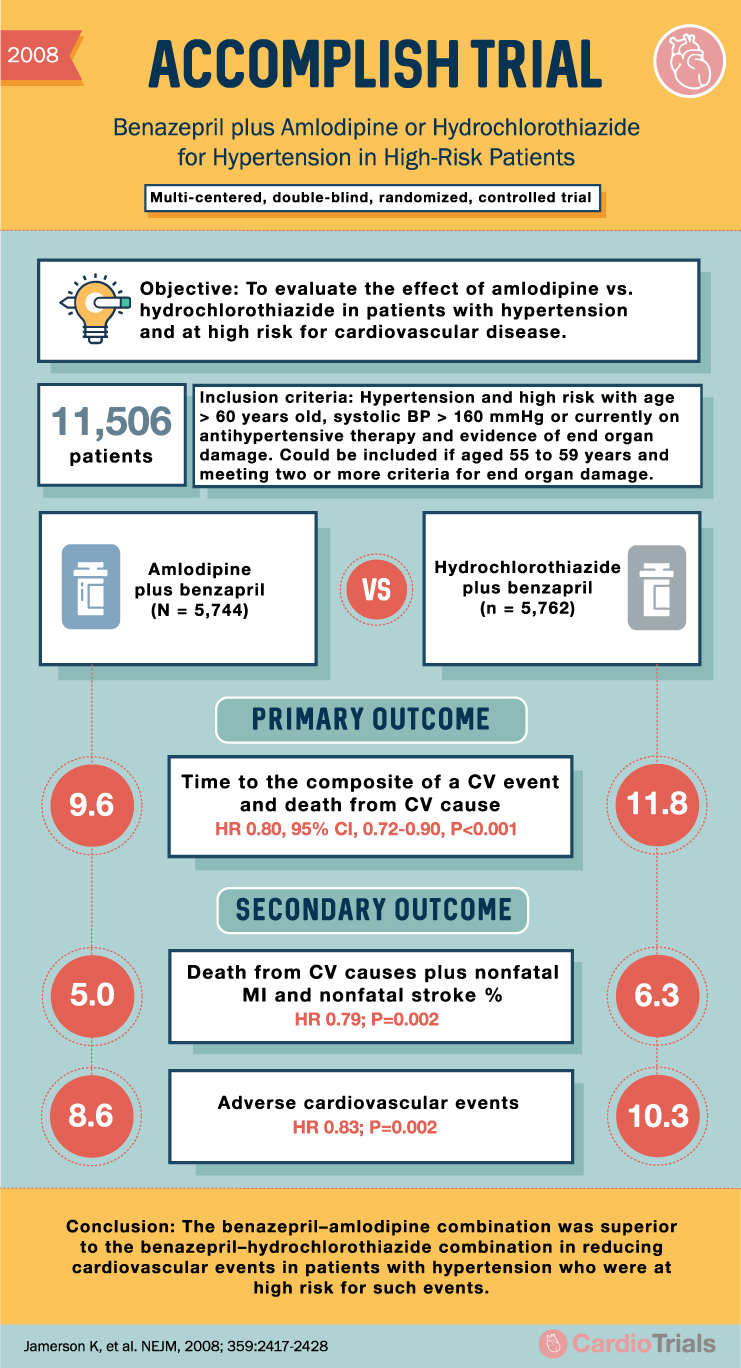
ACCOMPLISH Trial: Benazepril + Amlodipine or HCTZ for Hypertension
2008 ACCOMPLISH TRIAL Benazepril plus Amlodipine or Hydrochlorothiazide for Hypertension in High-Risk Patients Multi-centered, double-blind, randomized, controlled trial Objective: To evaluate the effect of amlodipine vs. hydrochlorothiazide in patients with hypertension and at high risk for cardiovascular disease. E 11,506 … Read More
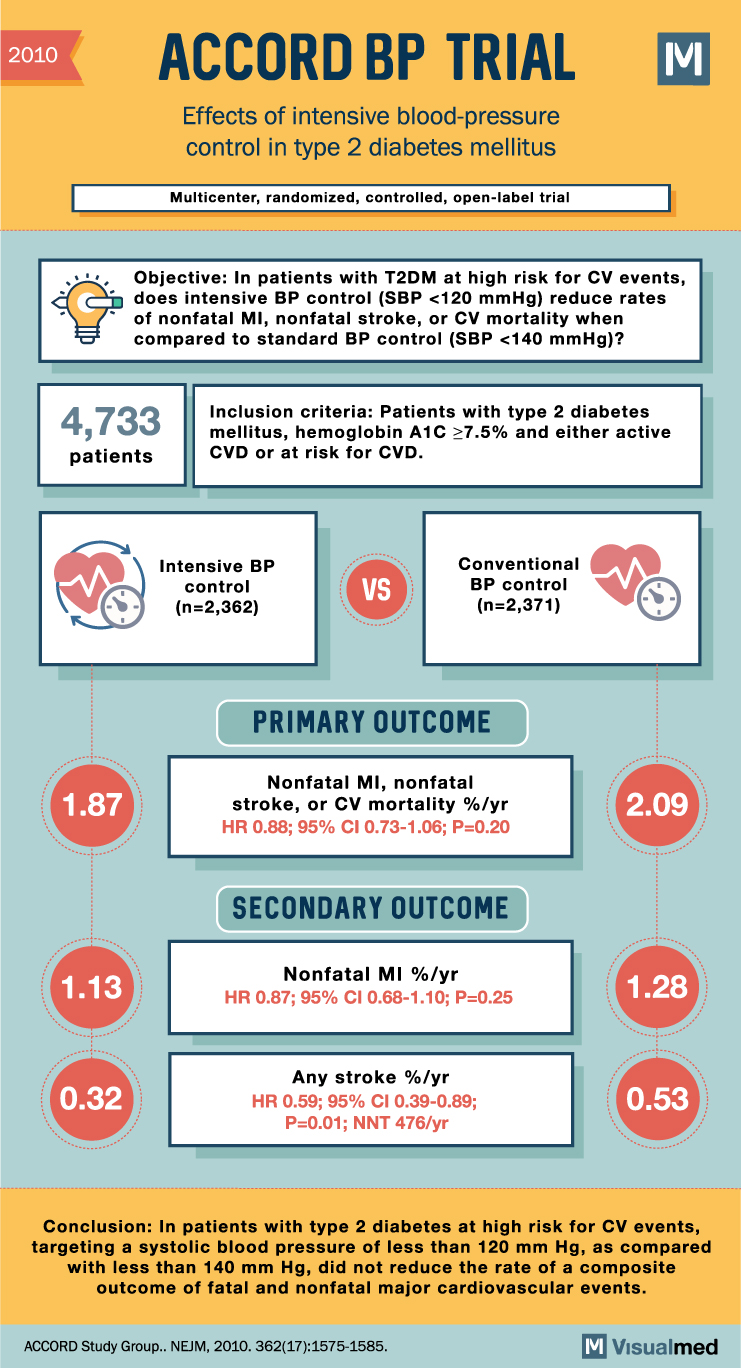
ACCORD BP Trial Summary: Intensive BP Control in Type 2 Diabetes
2010 ACCORD BP TRIAL Effects of intensive blood-pressure control in type 2 diabetes mellitus Multicenter, randomized, controlled, open-label trial Objective: In patients with T2DM at high risk for CV events, does intensive BP control (SBP <120 mmHg) reduce rates of … Read More
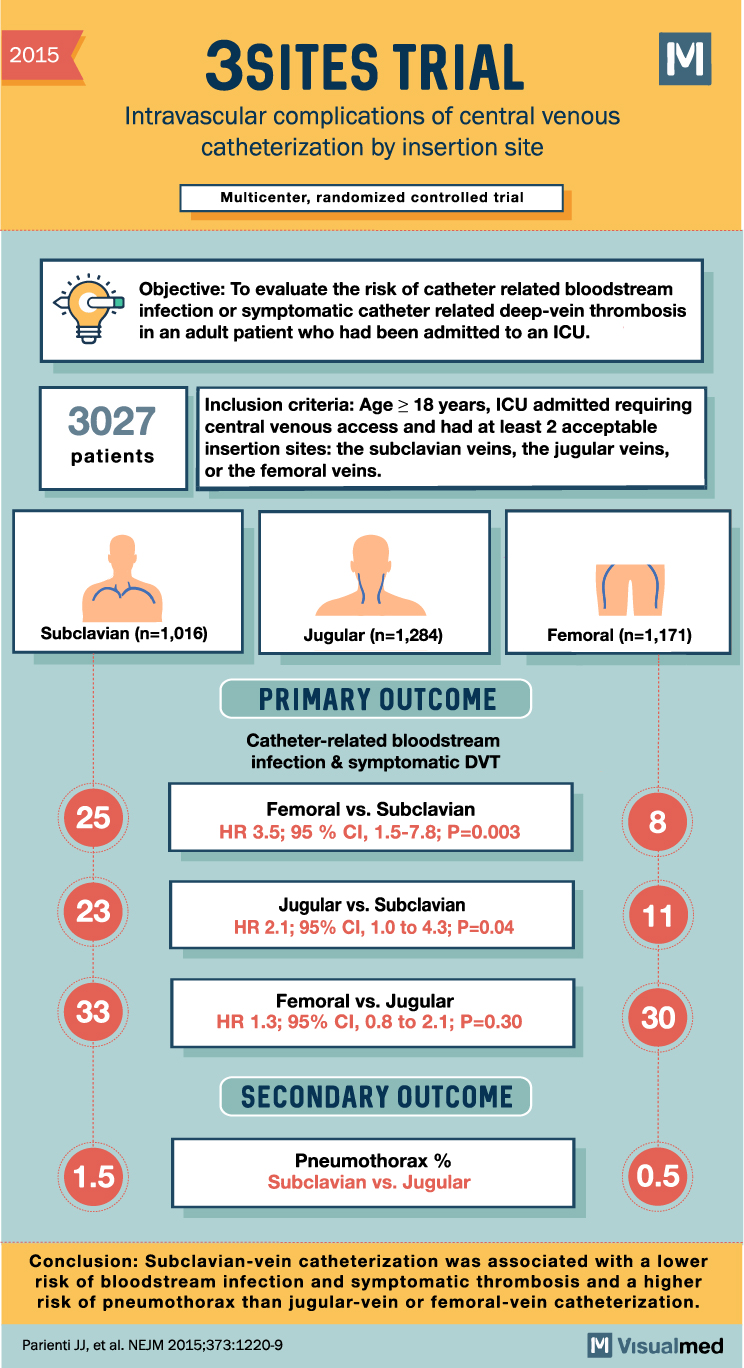
3SITES Trial Summary: Central Venous Catheterization Insertion Site
2015 3SITES TRIAL Intravascular complications of central venous catheterization by insertion site Multicenter, randomized controlled trial Objective: To evaluate the risk of catheter related bloodstream infection or symptomatic catheter related deep-vein thrombosis in an adult patient who had been admitted … Read More
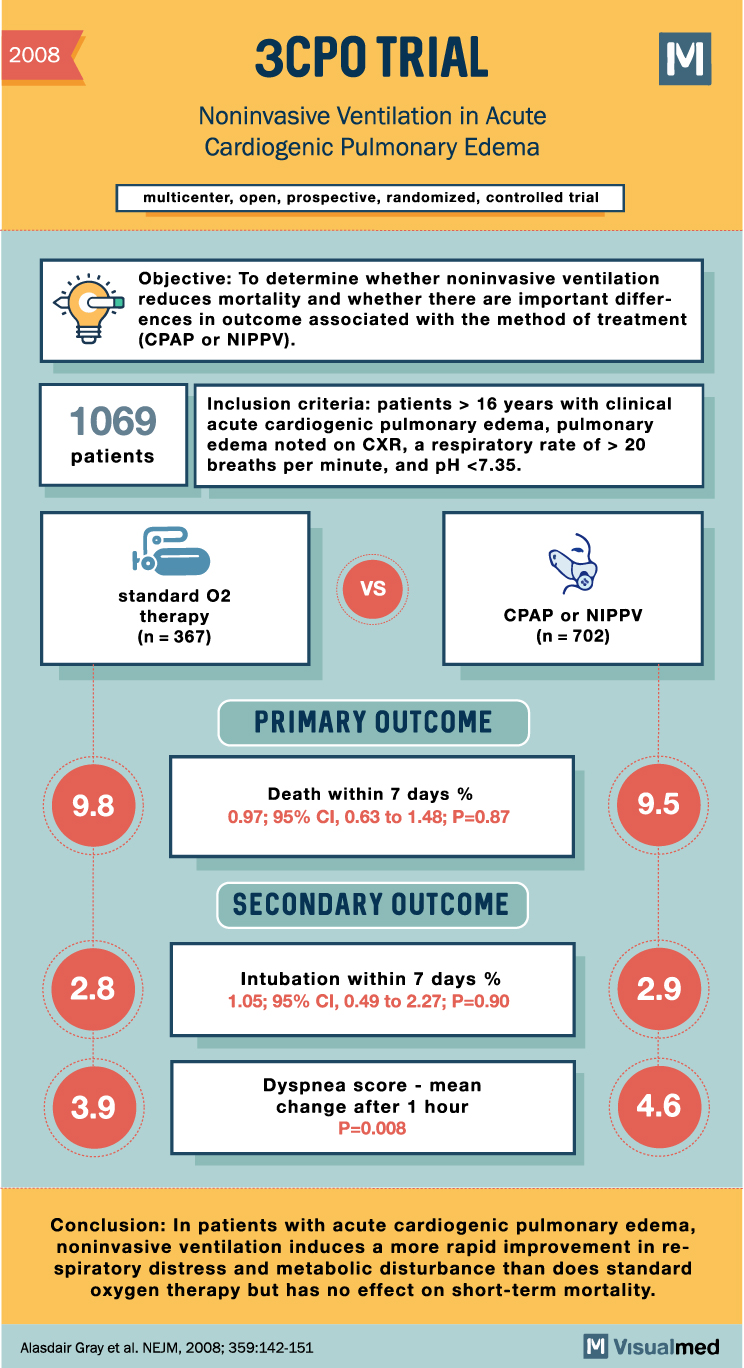
3CPO Trial Summary: Noninvasive Ventilation in Pulmonary Edema
2008 3CPO TRIAL Noninvasive Ventilation in Acute Cardiogenic Pulmonary Edema multicenter, open, prospective, randomized, controlled trial Objective: To determine whether noninvasive ventilation reduces mortality and whether there are important differences in outcome associated with the method of treatment (CPAP or … Read More
Visualmed App Update 2.9.9 – Upcoming New Features
Usama bin Nasir, MDFounder of Visualmed App
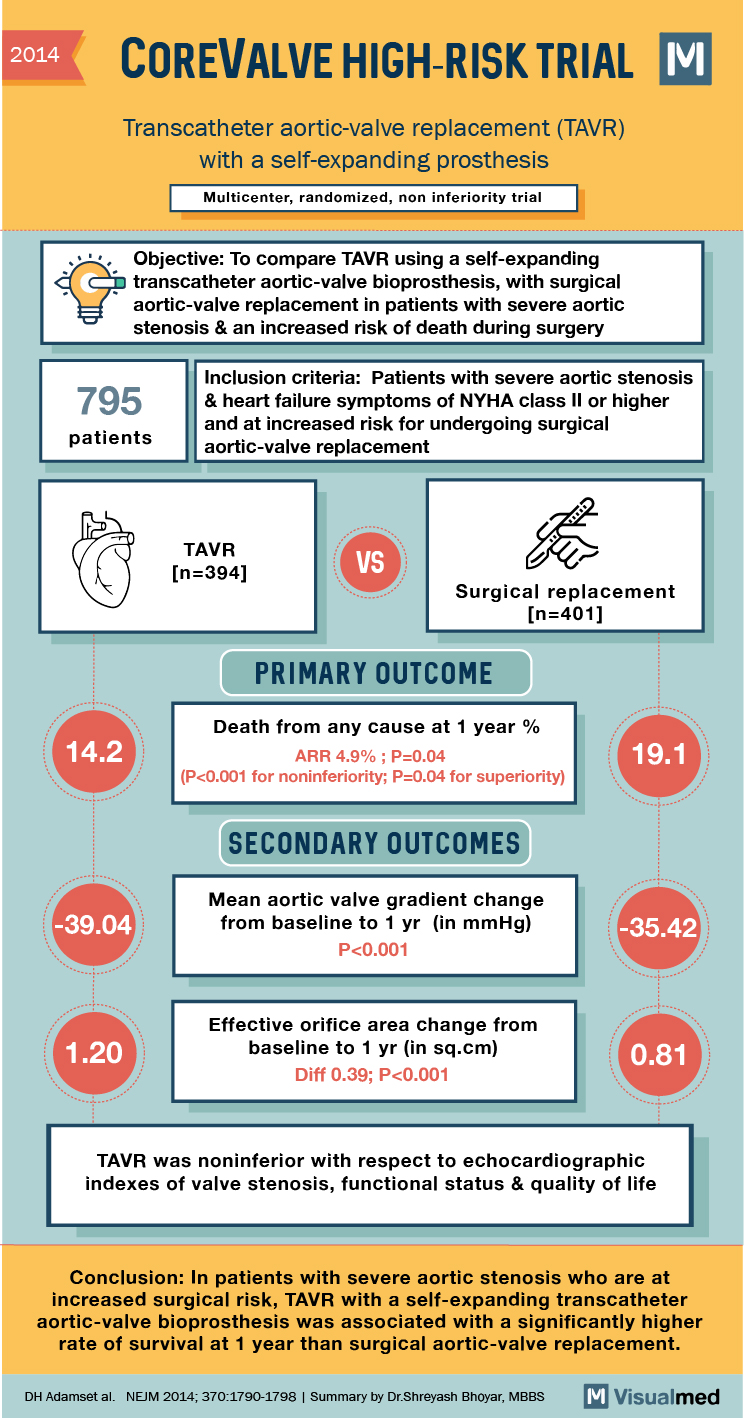
TAVR with Self-Expanding Prosthesis Trial Summary
2014 COREVALVE HIGH-RISK TRIAL Transcatheter aortic-valve replacement (TAVR) with a self-expanding prosthesis Multicenter, randomized, non inferiority trial “‘Objective: To compare TAVR using a self-expanding a transcatheter aortic-valve bioprosthesis, with surgical aortic-valve replacement in patients with severe aortic stenosis & an … Read More