Olezarsen for Managing Severe Hypertriglyceridemia and Pancreatitis Risk
Two double-blind, randomized, placebo-controlled phase 3 trials
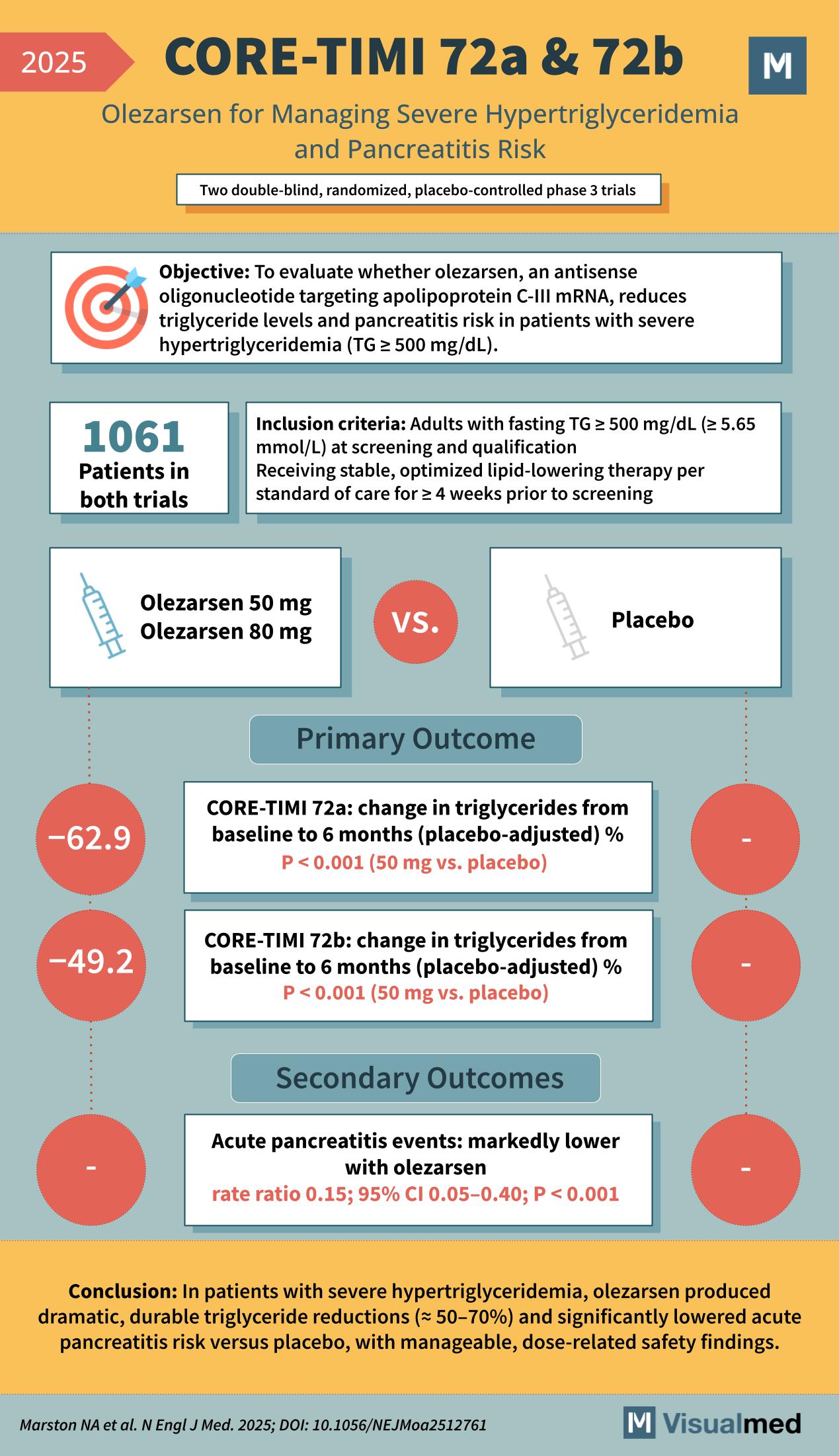
Objective:
To evaluate whether olezarsen, an antisense oligonucleotide targeting apolipoprotein C-III mRNA, reduces triglyceride levels and pancreatitis risk in patients with severe hypertriglyceridemia (TG ≥ 500 mg/dL).
Inclusion Criteria:
- Adults with fasting TG ≥ 500 mg/dL (≥ 5.65 mmol/L) at screening and qualification
- Receiving stable, optimized lipid-lowering therapy per standard of care for ≥ 4 weeks prior to screening
Population:
1,061 patients across two parallel trials
- CORE-TIMI 72a (n = 617)
- CORE2-TIMI 72b (n = 444)
Randomized 1:1:1 to monthly: - Olezarsen 50 mg
- Olezarsen 80 mg
- Placebo
Duration = 12 months
Primary Outcome:
Percent change in triglycerides from baseline to 6 months (placebo-adjusted):
| Trial | 50 mg | 80 mg |
|---|---|---|
| CORE-TIMI 72a | −62.9 pp | −72.2 pp |
| CORE2-TIMI 72b | −49.2 pp | −54.5 pp |
| (P < 0.001 for all vs placebo) |
Key Secondary Outcomes:
- Sustained TG reduction at 12 months
- Large decreases in Apo C-III, remnant cholesterol, and non-HDL cholesterol (P < 0.001 vs placebo)
- Acute pancreatitis events: markedly lower with olezarsen (rate ratio 0.15; 95% CI 0.05–0.40; P < 0.001)
Safety:
- Adverse-event rates similar to placebo overall
- 80 mg dose: more frequent mild ALT/AST elevations and thrombocytopenia (< 100 K/µL)
- Dose-related increase in hepatic fat fraction
Conclusion:
In patients with severe hypertriglyceridemia, olezarsen produced dramatic, durable triglyceride reductions (≈ 50–70%) and significantly lowered acute pancreatitis risk versus placebo, with manageable, dose-related safety findings.
Citation:
Marston NA et al. N Engl J Med. 2025; DOI: 10.1056/NEJMoa2512761