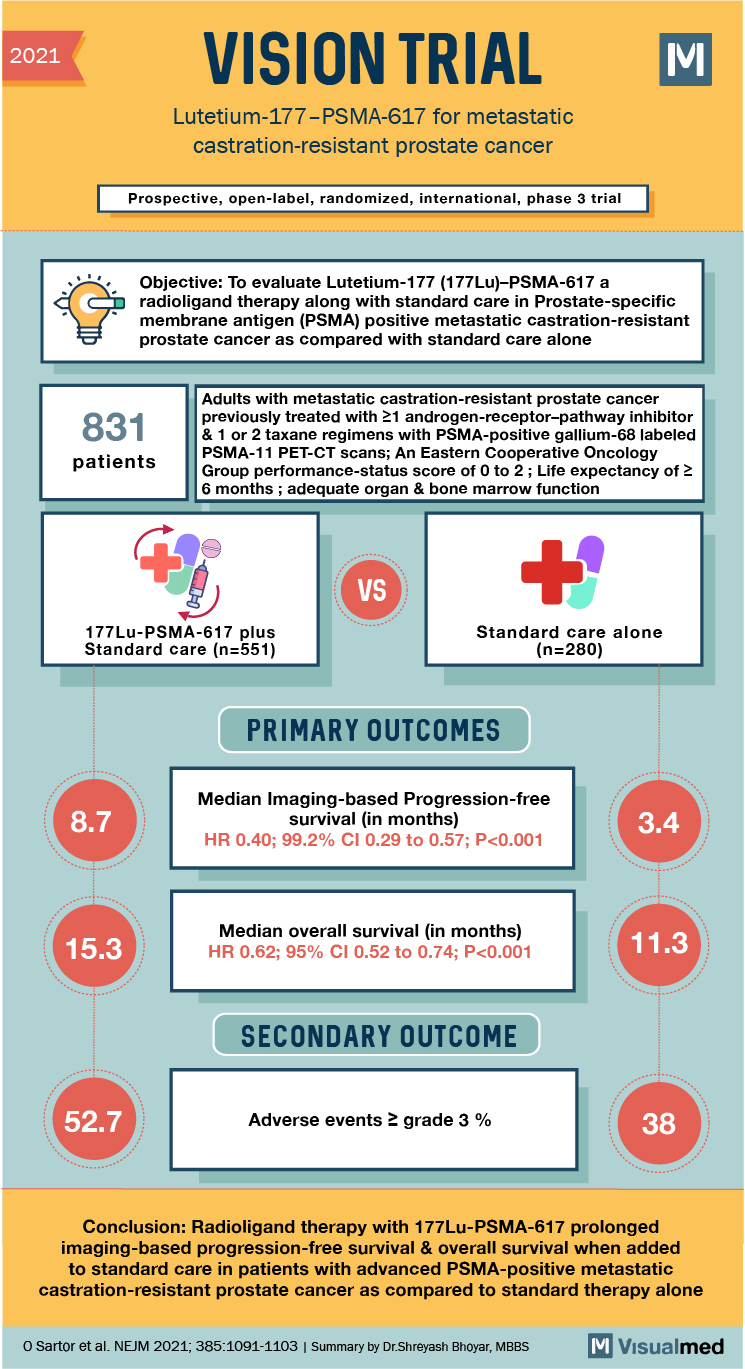
2021 VISION TRIAL Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer Prospective, open-label, randomized, international, phase 3 trial Objective: To evaluate Lutetium-177 (177Lu)-PSMA-617 a radioligand therapy along with standard care in Prostate-specific membrane antigen (PSMA) positive metastatic castration-resistant prostate cancer as compared with standard care alone 831 patients Adults with metastatic castration-resistant prostate cancer previously treated with 21 androgen-receptor-pathway inhibitor & 1 or 2 taxane regimens with PSMA-positive gallium-68 labeled PSMA-11 PET-CT scans; An Eastern Cooperative Oncology Group performance-status score of 0 to 2 ; Life expectancy of 2 6 months ; adequate organ & bone marrow function VS 177 Lu-PSMA-617 plus Standard care (n=551) Standard care alone (n=280) PRIMARY OUTCOMES 8.7 Median Imaging-based Progression-free survival (in months) HR 0.40; 99.2% CI 0.29 to 0.57; P<0.001 3.4 15.3 Median overall survival (in months) HR 0.62; 95% CI 0.52 to 0.74; P<0.001 SECONDARY OUTCOME 52.7 Adverse events 2 grade 3 % 38 Conclusion: Radioligand therapy with 177 Lu-PSMA-617 prolonged imaging-based progression-free survival & overall survival when added to standard care in patients with advanced PSMA-positive metastatic castration-resistant prostate cancer as compared to standard therapy alone O Sartor et al. NEJM 2021; 385:1091-1103