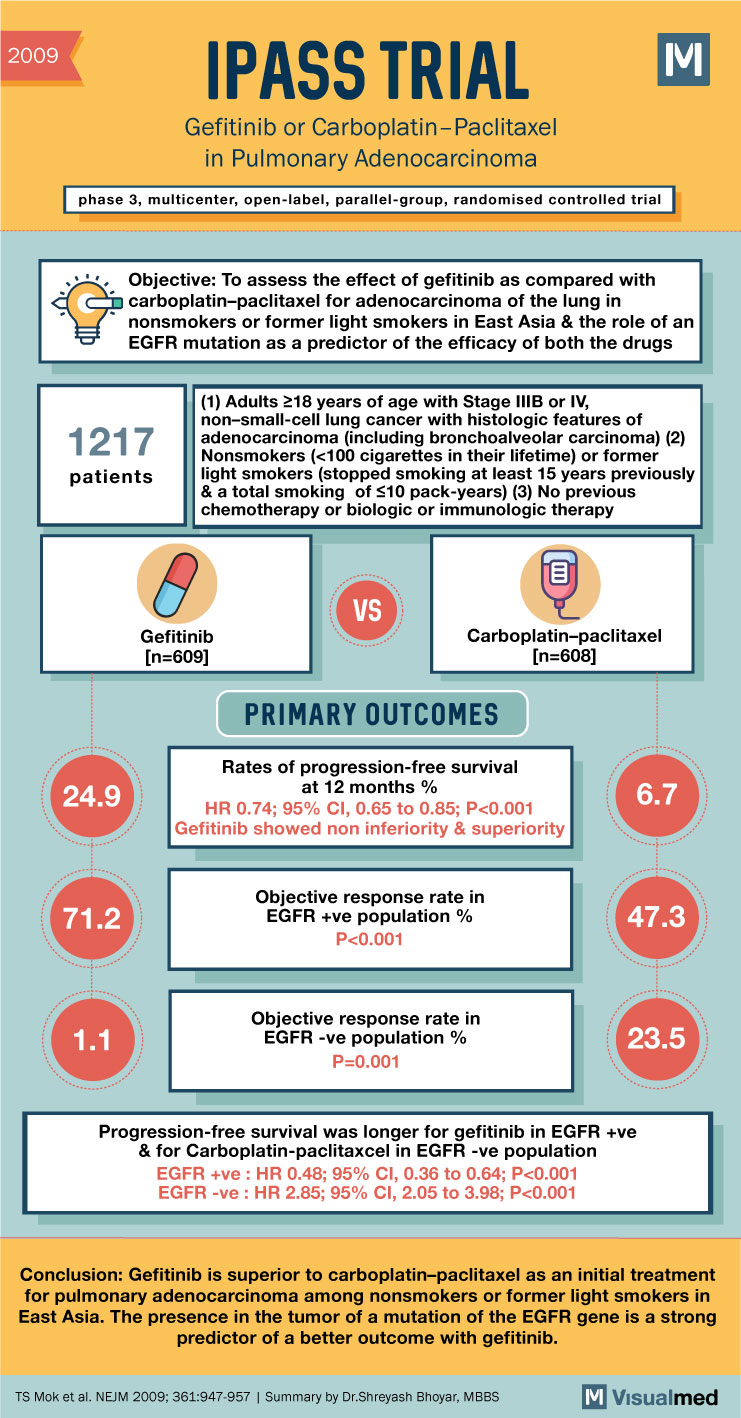
2009 IPASS TRIAL Gefitinib or Carboplatin-Paclitaxel in Pulmonary Adenocarcinoma phase 3, multicenter, open-label, parallel-group, randomised controlled trial d a Objective: To assess the effect of gefitinib as compared with carboplatin-paclitaxel for adenocarcinoma of the lung in nonsmokers or former light smokers in East Asia & the role of an EGFR mutation as a predictor of the efficacy of both the drugs 1217 (1) Adults 218 years of age with Stage IIIB or IV, non-small-cell lung cancer with histologic features of adenocarcinoma (including bronchoalveolar carcinoma) (2) Nonsmokers (<100 cigarettes in their lifetime) or former light smokers (stopped smoking at least 15 years previously & a total smoking of $10 pack-years) (3) No previous chemotherapy or biologic or immunologic therapy patients VS Gefitinib (n=609] Carboplatin-paclitaxel- PRIMARY OUTCOMES 24.9 Rates of progression-free survival at 12 months % HR 0.74; 95% CI, 0.65 to 0.85; P<0.001 Gefitinib showed non inferiority & superiority 6.7 71.2 Objective response rate in EGFR +ve population % P<0.001 47.3 1.1 Objective response rate in EGFR -ve population % P=0.001 23.5 Progression-free survival was longer for gefitinib in EGFR +ve & for Carboplatin-paclitaxcel in EGFR -ve population EGFR +ve: HR 0.48; 95% CI, 0.36 to 0.64; P<0.001 EGFR -ve : HR 2.85; 95% CI, 2.05 to 3.98; P<0.001 Conclusion: Gefitinib is superior to carboplatin-paclitaxel as an initial treatment for pulmonary adenocarcinoma among nonsmokers or former light smokers in East Asia. The presence in the tumor of a mutation of the EGFR gene is a strong predictor of a better outcome with gefitinib. TS Mok et al. NEJM 2009; 361:947-957