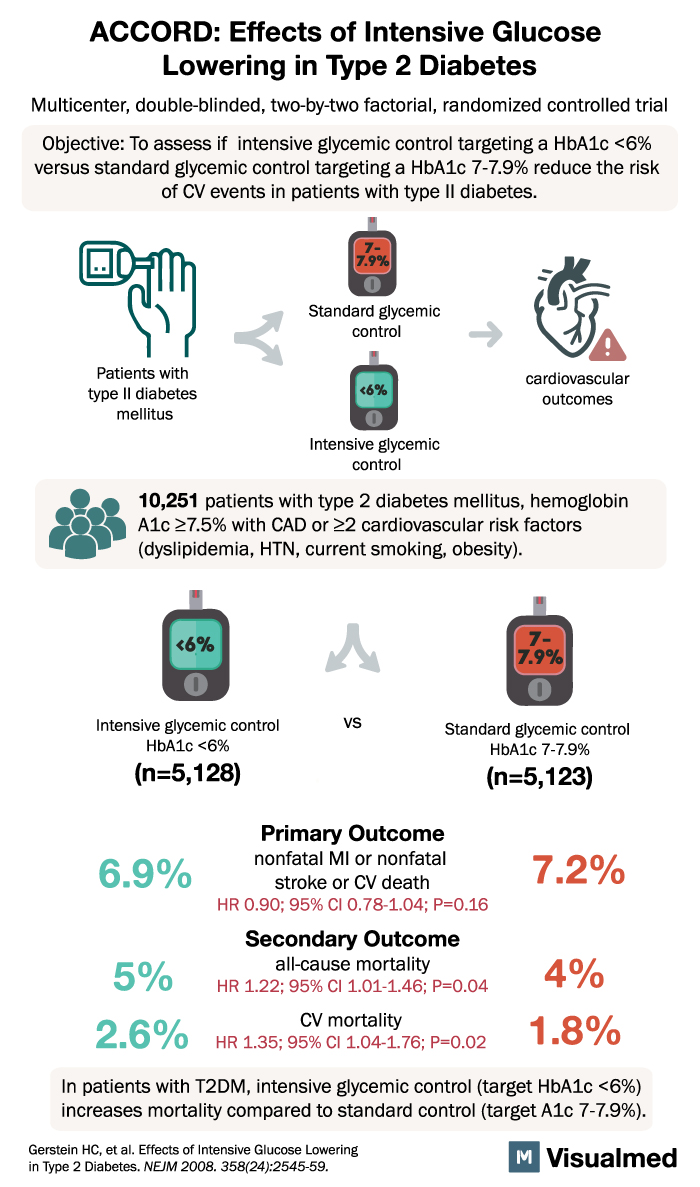
ACCORD: Effects of Intensive Glucose Lowering in Type 2 Diabetes Multicenter, double-blinded, two-by-two factorial, randomized controlled trial Objective: To assess if intensive glycemic control targeting a HbA1c <6% versus standard glycemic control targeting a HbA1c 7-7.9% reduce the risk of CV events in patients with type II diabetes. Patients with type II diabetes mellitus 7.9% 0 Standard glycemic control <6% cardiovascular outcomes Intensive glycemic control 10,251 patients with type 2 diabetes mellitus, hemoglobin A1c ≥7.5% with CAD or ≥2 cardiovascular risk factors (dyslipidemia, HTN, current smoking, obesity). <6% 7- 7.9% Intensive glycemic control HbA1c <6% VS Standard glycemic control HbA1c 7-7.9% 6.9% 5% 2.6% (n=5,128) Primary Outcome nonfatal MI or nonfatal stroke or CV death HR 0.90; 95% CI 0.78-1.04; P=0.16 Secondary Outcome all-cause mortality HR 1.22; 95% CI 1.01-1.46; P=0.04 (n=5,123) 7.2% CV mortality 4% 1.8% HR 1.35; 95% CI 1.04-1.76; P=0.02 In patients with T2DM, intensive glycemic control (target HbA1c <6%) increases mortality compared to standard control (target A1c 7-7.9%). Gerstein HC, et al. Effects of Intensive Glucose Lowering in Type 2 Diabetes. NEJM 2008. 358(24):2545-59.