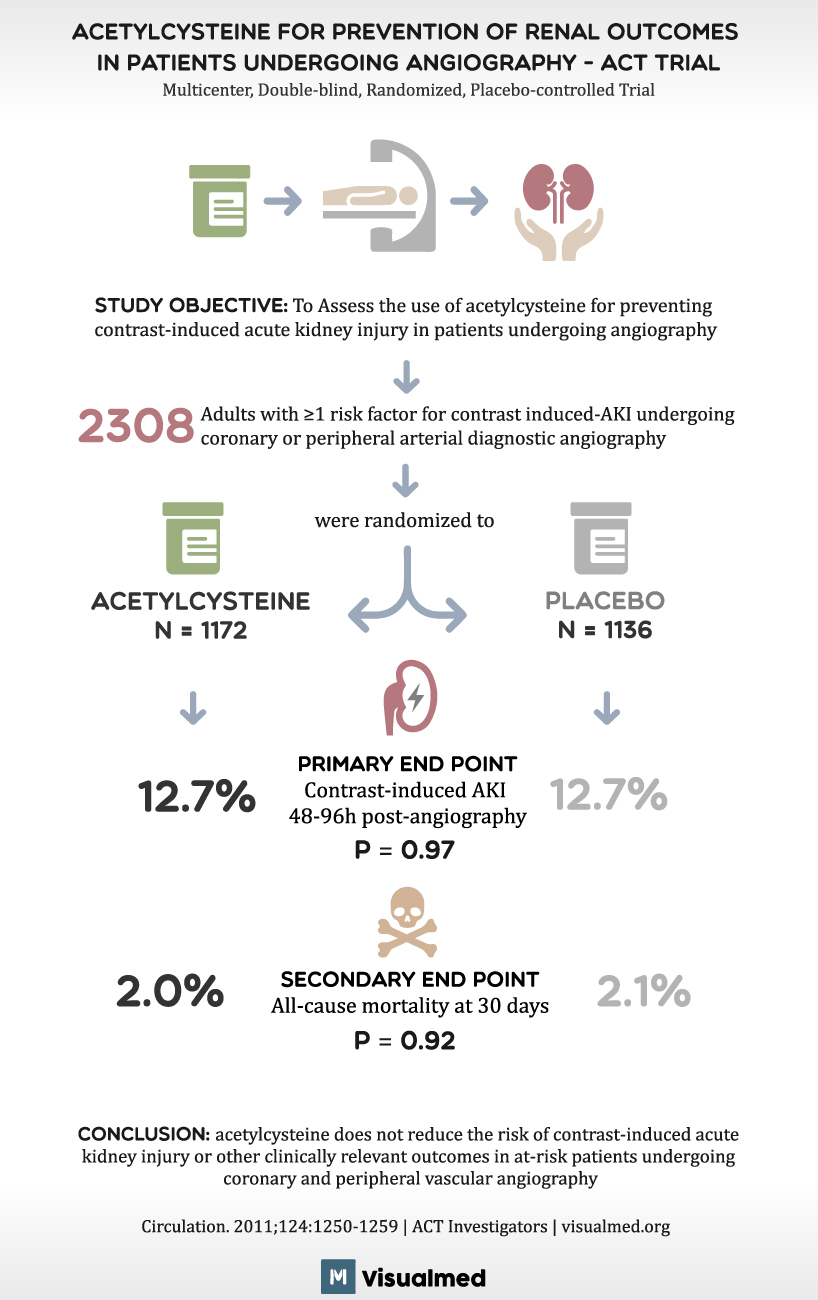
ACETYLCYSTEINE FOR PREVENTION OF RENAL OUTCOMES IN PATIENTS UNDERGOING ANGIOGRAPHY – ACT TRIAL Multicenter, Double-blind, Randomized, Placebo-controlled Trial STUDY OBJECTIVE: To Assess the use of acetylcysteine for preventing contrast-induced acute kidney injury in patients undergoing angiography 2308 Adults with ≥1 risk factor for contrast induced-AKI undergoing coronary or peripheral arterial diagnostic angiography ACETYLCYSTEINE N = 1172 12.7% 2.0% were randomized to يله PRIMARY END POINT Contrast-induced AKI 48-96h post-angiography P = 0.97 SECONDARY END POINT All-cause mortality at 30 days P = 0.92 PLACEBO N = 1136 12.7% 2.1% CONCLUSION: acetylcysteine does not reduce the risk of contrast-induced acute kidney injury or other clinically relevant outcomes in at-risk patients undergoing coronary and peripheral vascular angiography Circulation. 2011;124:1250-1259 | ACT Investigators |