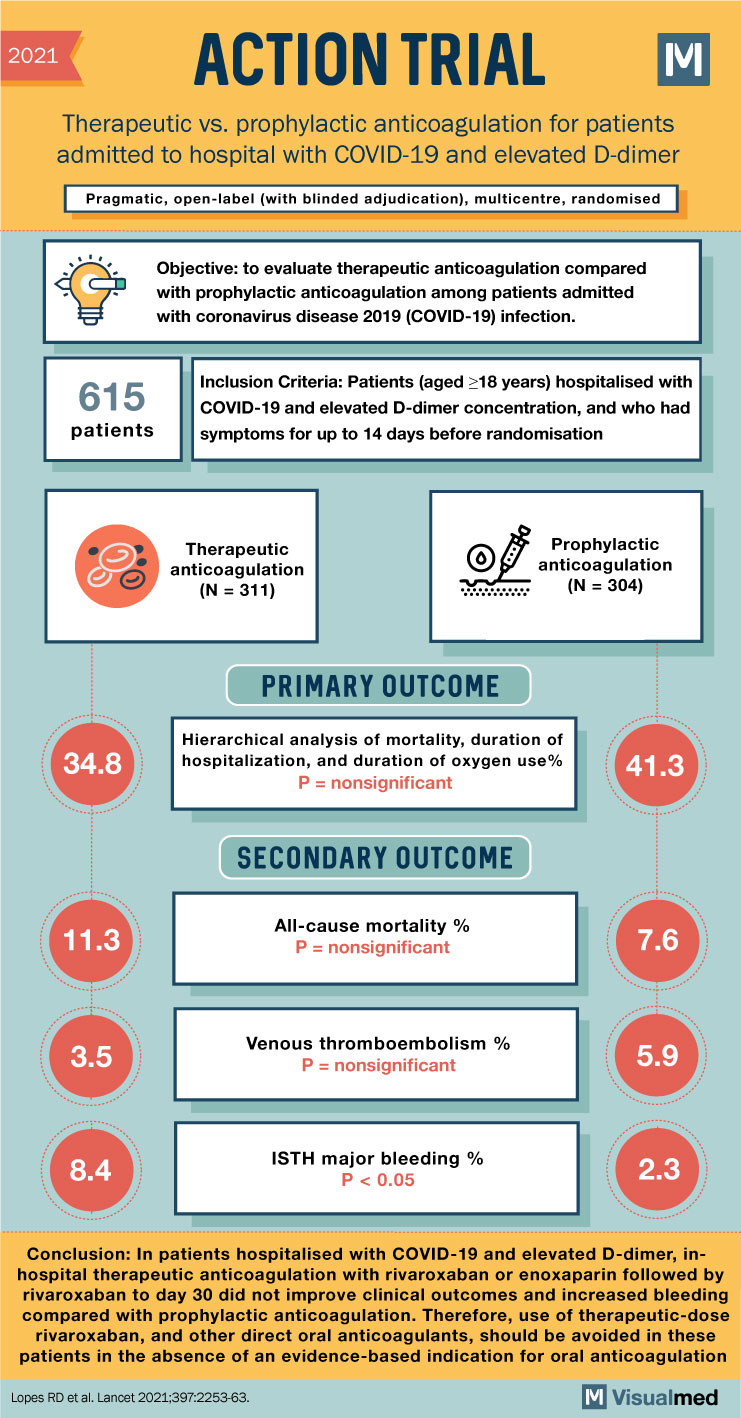
2021 ACTION TRIAL Therapeutic vs. prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer Pragmatic, open-label (with blinded adjudication), multicentre, randomised Objective: to evaluate therapeutic anticoagulation compared with prophylactic anticoagulation among patients admitted with coronavirus disease 2019 (COVID-19) infection. 615 patients Inclusion Criteria: Patients (aged >18 years) hospitalised with COVID-19 and elevated D-dimer concentration, and who had symptoms for up to 14 days before randomisation Therapeutic anticoagulation (N = 311) Prophylactic anticoagulation (N = 304) . PRIMARY OUTCOME 34.8 Hierarchical analysis of mortality, duration of hospitalization, and duration of oxygen use% P = nonsignificant 41.3 SECONDARY OUTCOME 11.3 All-cause mortality % P = nonsignificant 7.6 3.5 Venous thromboembolism % P = nonsignificant 5.9 8.4 ISTH major bleeding % P<0.05 2.3 Conclusion: In patients hospitalised with COVID-19 and elevated D-dimer, inhospital therapeutic anticoagulation with rivaroxaban or enoxaparin followed by rivaroxaban to day 30 did not improve clinical outcomes and increased bleeding compared with prophylactic anticoagulation. Therefore, use of therapeutic-dose rivaroxaban, and other direct oral anticoagulants, should be avoided in these patients in the absence of an evidence-based indication for oral anticoagulation Lopes RD et al. Lancet 2021:397:2253-63.