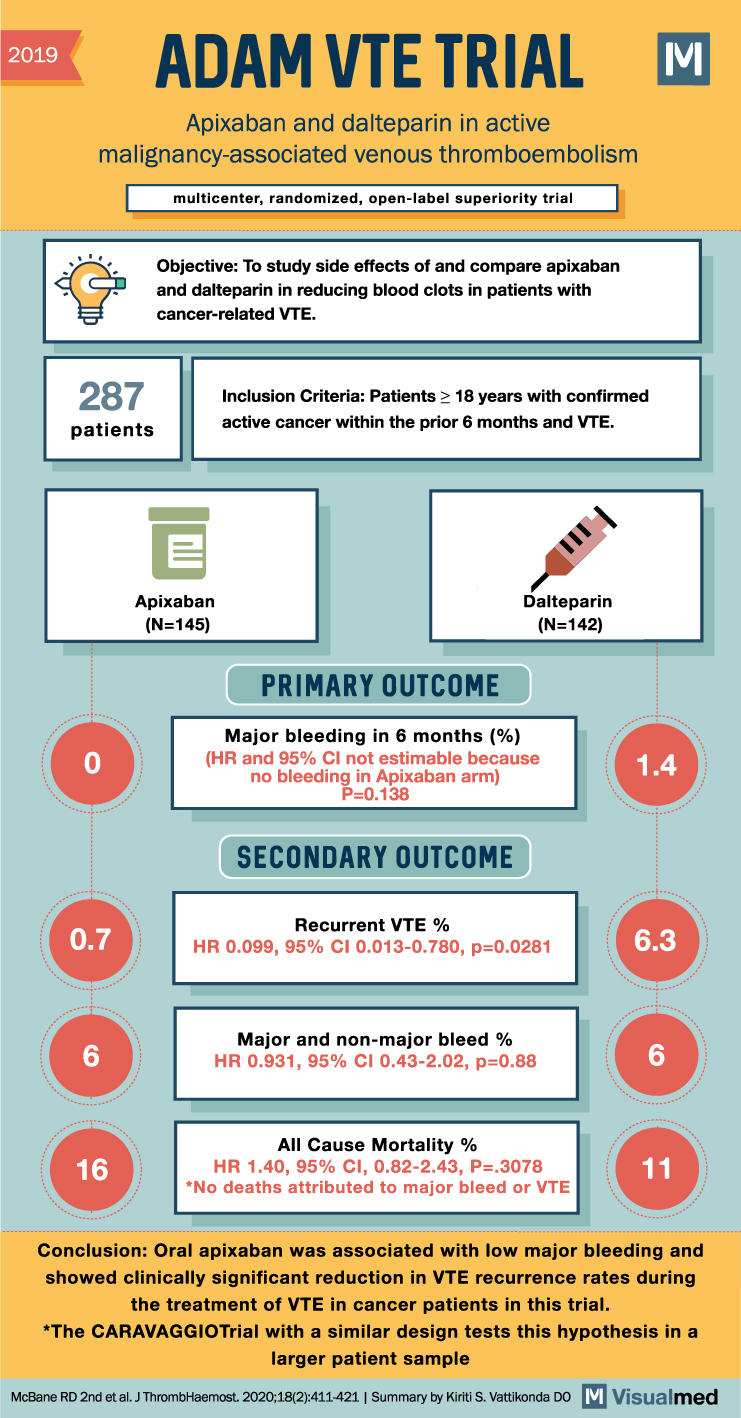
2019 ADAM VTE TRIAL Apixaban and dalteparin in active malignancy-associated venous thromboembolism multicenter, randomized, open-label superiority trial 287 patients 0 Objective: To study side effects of and compare apixaban and dalteparin in reducing blood clots in patients with cancer-related VTE. Inclusion Criteria: Patients ≥ 18 years with confirmed active cancer within the prior 6 months and VTE. Dalteparin (N=142) Apixaban (N=145) PRIMARY OUTCOME Major bleeding in 6 months (%) (HR and 95% CI not estimable because no bleeding in Apixaban arm) P=0.138 SECONDARY OUTCOME 1.4 Recurrent VTE % 0.7 HR 0.099, 95% CI 0.013-0.780, p=0.0281 6.3 Major and non-major bleed % 6 HR 0.931, 95% CI 0.43-2.02, p=0.88 6 All Cause Mortality % 16 HR 1.40, 95% CI, 0.82-2.43, P=.3078 *No deaths attributed to major bleed or VTE 11 Conclusion: Oral apixaban was associated with low major bleeding and showed clinically significant reduction in VTE recurrence rates during the treatment of VTE in cancer patients in this trial. *The CARAVAGGIOTrial with a similar design tests this hypothesis in a larger patient sample McBane RD 2nd et al. J Thromb Haemost. 2020;18(2):411-421