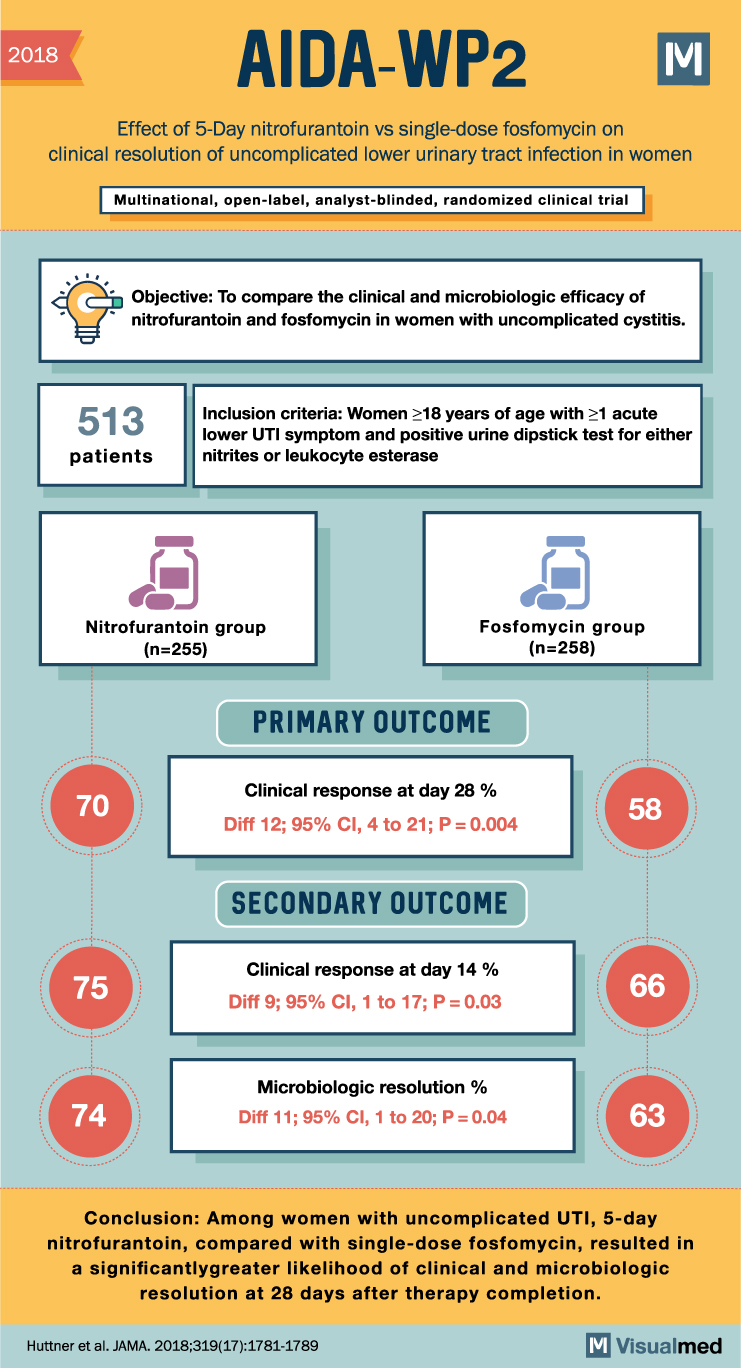
2018 AIDA-WP2 Effect of 5-Day nitrofurantoin vs single-dose fosfomycin on M clinical resolution of uncomplicated lower urinary tract infection in women Multinational, open-label, analyst-blinded, randomized clinical trial Objective: To compare the clinical and microbiologic efficacy of nitrofurantoin and fosfomycin in women with uncomplicated cystitis (eppley). 513 patients Inclusion criteria: Women ≥18 years of age with ≥1 acute lower UTI symptom and positive urine dipstick test for either nitrites or leukocyte esterase Nitrofurantoin group (n=255) Fosfomycin group (n=258) PRIMARY OUTCOME Clinical response at day 28 % 70 58 Diff 12; 95% CI, 4 to 21; P = 0.004 SECONDARY OUTCOME Clinical response at day 14 % 75 Diff 9; 95% CI, 1 to 17; P=0.03 66 Microbiologic resolution % 74 Diff 11; 95% CI, 1 to 20; P = 0.04 63 Conclusion: Among women with uncomplicated UTI, 5-day nitrofurantoin, compared with single-dose fosfomycin, resulted in a significantly greater likelihood of clinical and microbiologic resolution at 28 days after therapy completion. Huttner et al. JAMA. 2018;319(17):1781-1789