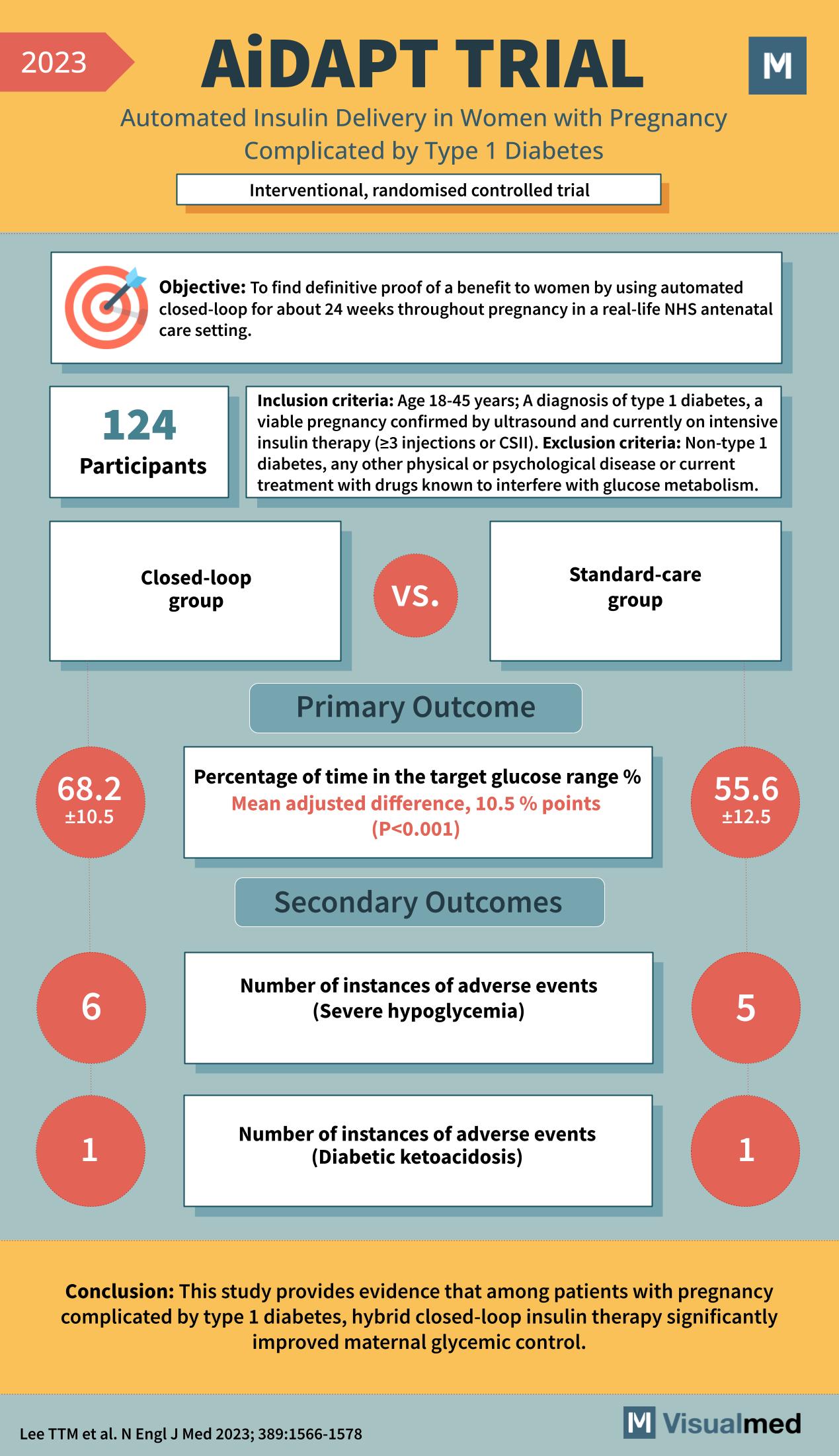
Year: 2023 Title: AiDAPT TRIAL Subtitle: Automated Insulin Delivery in Women with Pregnancy Complicated by Type 1 Diabetes Type of Trial: Interventional, randomized controlled trial
Objective: To find definitive proof of a benefit to women by using automated closed-loop for about 24 weeks throughout pregnancy in a real-life NHS antenatal care setting.
Participants: 124
Inclusion Criteria:
- Age 18-45 years
- A diagnosis of type 1 diabetes
- A viable pregnancy confirmed by ultrasound and currently on intensive insulin therapy (≥3 injections or CSII)
Exclusion Criteria:
- Non-type 1 diabetes
- Any other physical or psychological disease or current treatment with drugs known to interfere with glucose metabolism.
Groups:
- Closed-loop group
- Standard-care group
Primary Outcome:
- Percentage of time in the target glucose range %
- Closed-loop group: 68.2 ±10.5
- Standard-care group: 55.6 ±12.5
- Mean adjusted difference, 10.5 percentage points (P<0.001)
Secondary Outcomes:
- Number of instances of adverse events (Severe hypoglycemia)
- Closed-loop group: 6
- Standard-care group: 5
- Number of instances of adverse events (Diabetic ketoacidosis)
- Closed-loop group: 1
- Standard-care group: 1
Conclusion: This study provides evidence that among patients with pregnancy complicated by type 1 diabetes, hybrid closed-loop insulin therapy significantly improved maternal glycemic control.
Reference: Lee TTM et al. N Engl J Med 2023; 389:1566-1578