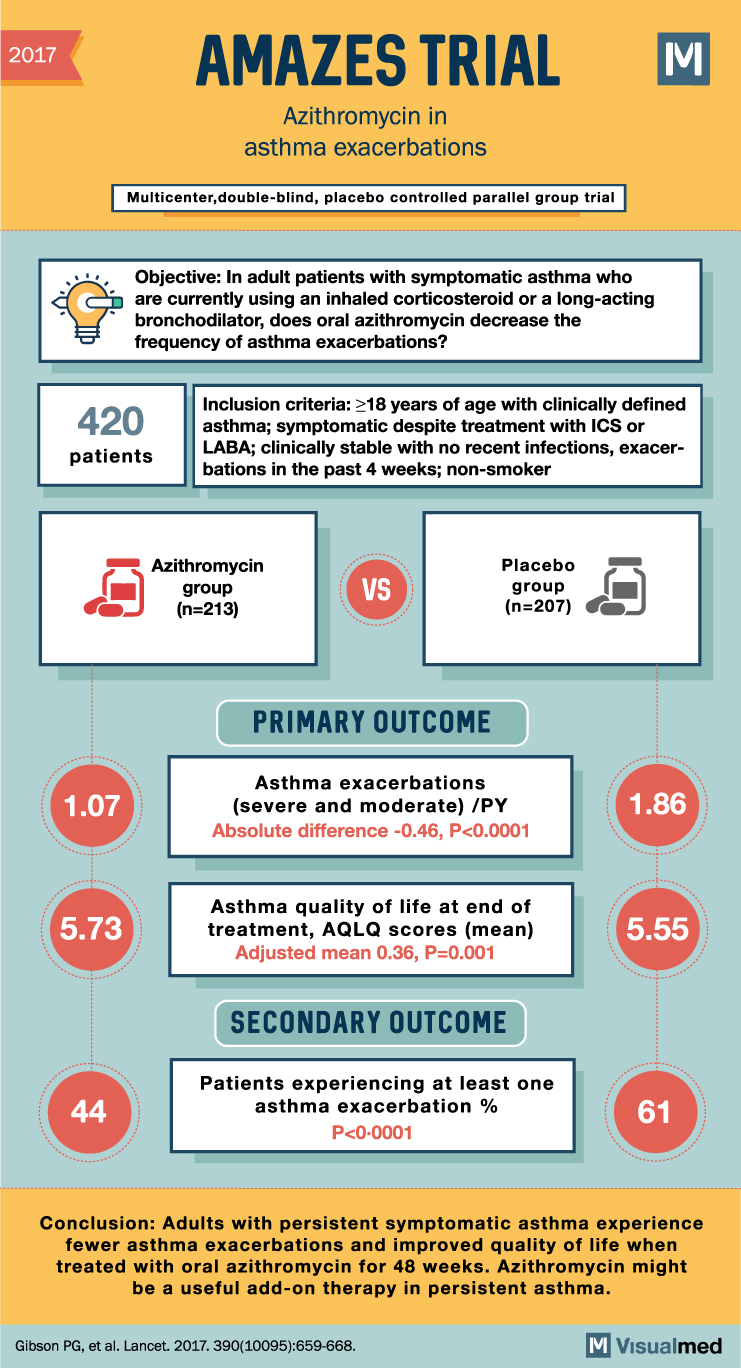
2017 AMAZES TRIAL Azithromycin in asthma exacerbations Multicenter, double-blind, placebo controlled parallel group trial M Objective: In adult patients with symptomatic asthma who are currently using an inhaled corticosteroid or a long-acting bronchodilator, does oral azithromycin decrease the frequency of asthma exacerbations? 420 patients Inclusion criteria: ≥18 years of age with clinically defined asthma; symptomatic despite treatment with ICS or LABA; clinically stable with no recent infections, exacer- bations in the past 4 weeks; non-smoker Azithromycin Placebo group (n=213) VS group (n=207) PRIMARY OUTCOME Asthma exacerbations 1.07 (severe and moderate) /PY Absolute difference -0.46, P<0.0001 1.86 5.73 Asthma quality of life at end of treatment, AQLQ scores (mean) Adjusted mean 0.36, P=0.001 5.55 44 SECONDARY OUTCOME Patients experiencing at least one asthma exacerbation % P<0.0001 61 Conclusion: Adults with persistent symptomatic asthma experience fewer asthma exacerbations and improved quality of life when treated with oral azithromycin for 48 weeks. Azithromycin might be a useful add-on therapy in persistent asthma. Gibson PG, et al. Lancet. 2017. 390(10095):659-668.