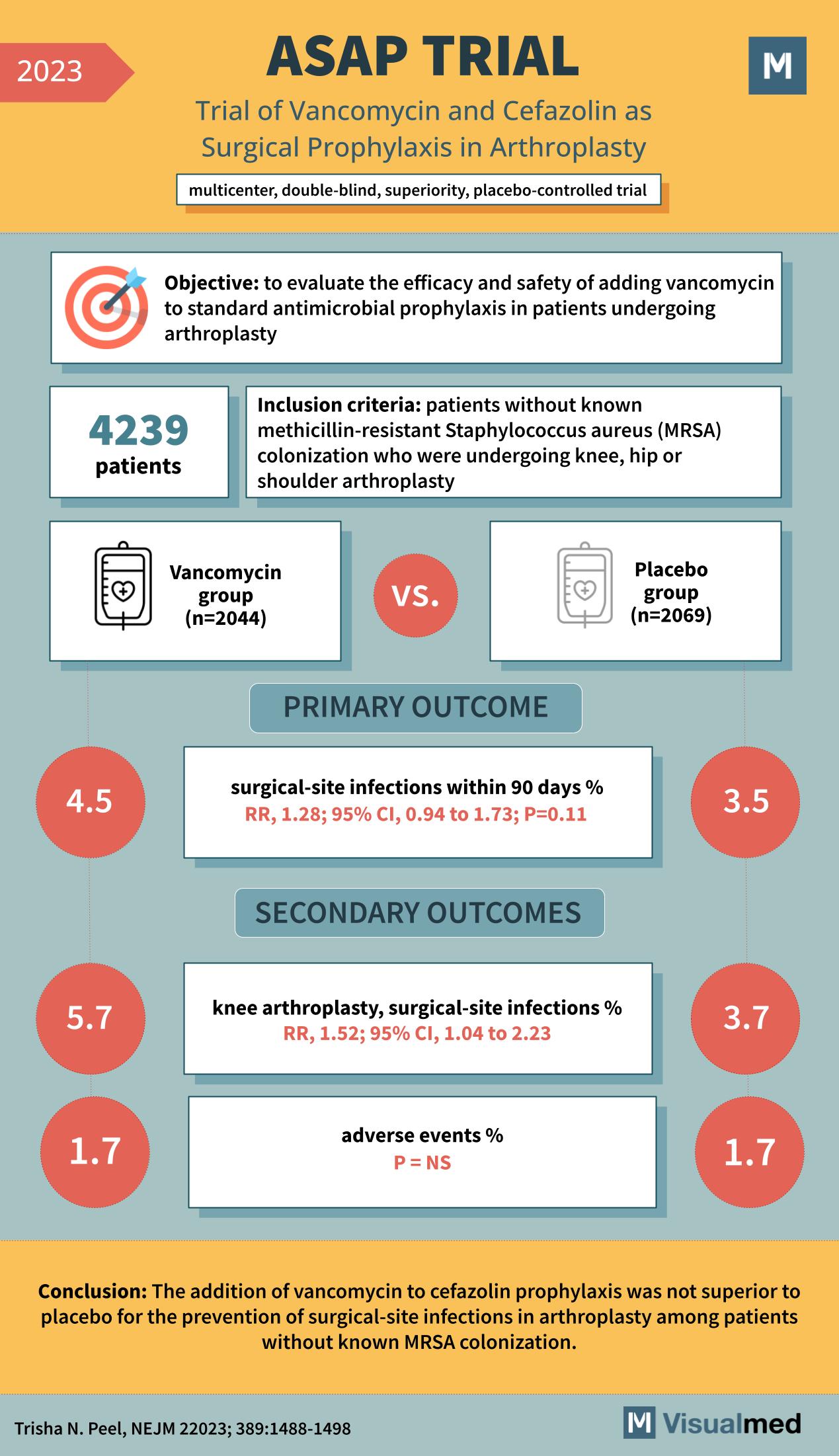
The ASAP Trial: Vancomycin and Cefazolin in Arthroplasty Prophylaxis – An Analysis The realm of surgical prophylaxis has always been a topic of deep medical interest, aiming to prevent complications and ensure the best outcomes for patients. One such endeavor is the ASAP Trial of 2023, a multicenter, double-blind, superiority, placebo-controlled study. This trial sought to explore the efficacy of adding vancomycin to the standard antimicrobial prophylaxis in patients undergoing arthroplasty.
Objective and Methodology
Arthroplasty, a surgical procedure to restore the function of a joint, is commonly performed on the knee, hip, or shoulder. One of the critical concerns in such surgeries is the prevention of surgical-site infections. The ASAP Trial’s primary objective was to evaluate the safety and efficacy of adding vancomycin to standard antimicrobial prophylaxis for these patients. The study included 4239 patients who were undergoing knee, hip, or shoulder arthroplasty without known colonization of methicillin-resistant Staphylococcus aureus (MRSA). The participants were divided into two groups: the Vancomycin group (n=2044) and the Placebo group (n=2069).
Key Outcomes
The primary outcome measured was the percentage of surgical-site infections within 90 days post-operation. The results showed that the Vancomycin group had a 4.5% infection rate, compared to the 3.5% in the Placebo group. This yielded a relative risk (RR) of 1.28, with a 95% confidence interval (CI) of 0.94 to 1.73, and a p-value of 0.11. While the infection rate was higher in the Vancomycin group, the difference was not statistically significant. For the secondary outcomes, a subset analysis was performed specifically on knee arthroplasty patients. Here, the surgical-site infection rate was 5.7% in the Vancomycin group and 3.7% in the Placebo group. The RR for this subset was 1.52, with a 95% CI of 1.04 to 2.23. Another crucial outcome was the percentage of adverse events post-operation. Both the Vancomycin and Placebo groups demonstrated an equal adverse event percentage of 1.7%.
Conclusion
The addition of vancomycin to cefazolin prophylaxis was not found to be superior to placebo for preventing surgical-site infections in arthroplasty among patients without known MRSA colonization. This is a pivotal finding, as it questions the benefit of adding vancomycin to the standard prophylactic regimen in this context.
Implications for the Medical Community
While the intent behind adding an antibiotic like vancomycin would presumably be to bolster the defense against potential infections, the ASAP Trial shows that this might not always yield the expected outcomes. This finding reminds clinicians of the importance of evidence-based practice and the necessity of continuous research in ensuring the best patient care. In conclusion, the ASAP Trial offers valuable insights into surgical prophylaxis for arthroplasty, emphasizing the need for a more nuanced approach in selecting antibiotic regimens and highlighting the value of focused clinical trials in guiding medical practice.