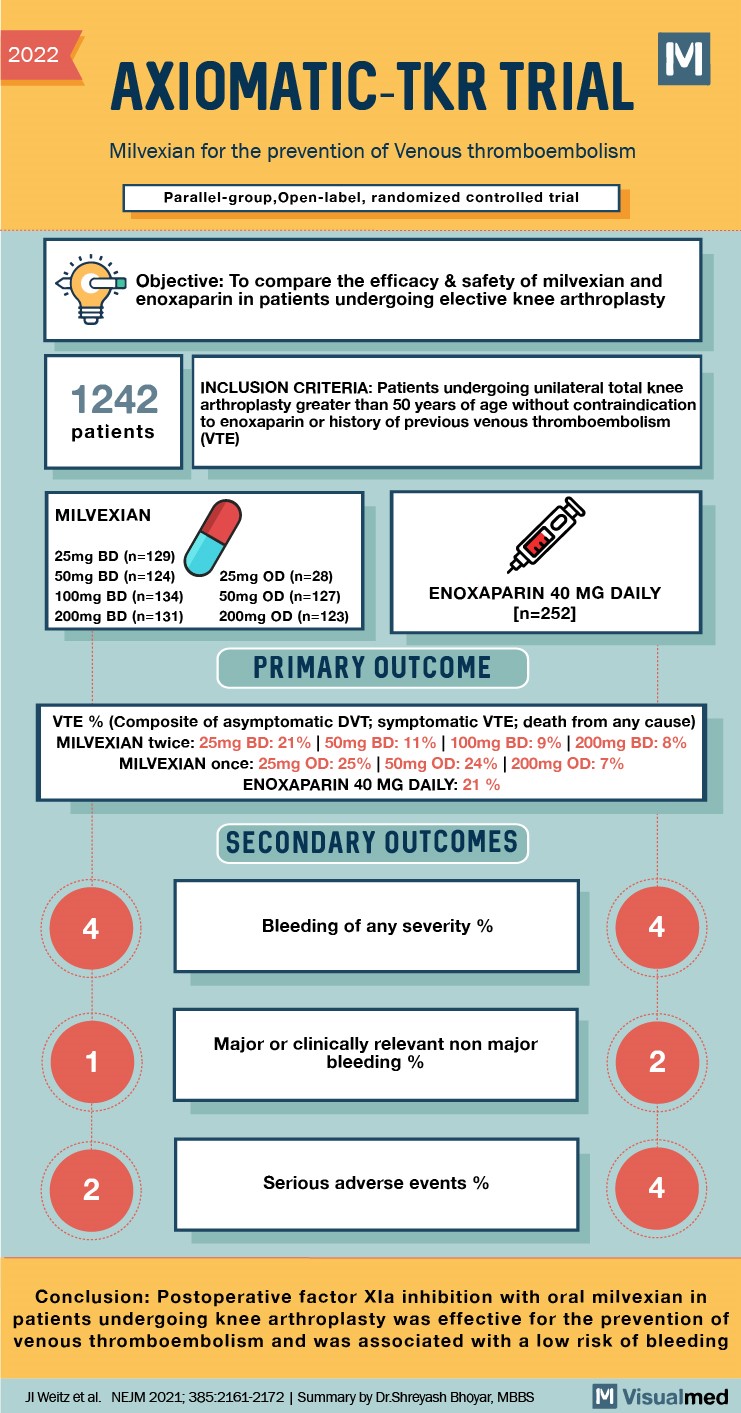
2022 AXIOMATIC-TKR TRIAL Milvexian for the prevention of Venous thromboembolism Parallel-group, Open-label, randomized controlled trial Objective: To compare the efficacy & safety of milvexian and enoxaparin in patients undergoing elective knee arthroplasty 1242 patients INCLUSION CRITERIA: Patients undergoing unilateral total knee arthroplasty greater than 50 years of age without contraindicatior to enoxaparin or history of previous venous thromboembolism (VTE) MILVEXIAN 25mg BD (n=129) 50mg BD (n=124) 100mg BD (n=134) 200mg BD (n=131) 25mg OD (n=28) 50mg OD (n=127) 200mg OD (n=123) ENOXAPARIN 40 MG DAILY [n=252] PRIMARY OUTCOME VTE % (Composite of asymptomatic DVT; symptomatic VTE; death from any cause) MILVEXIAN twice: 25mg BD: 21% | 50mg BD: 11% 100mg BD: 9% 200mg BD: 8% MILVEXIAN once: 25mg OD: 25% 50mg OD: 24% 200mg OD: 7% ENOXAPARIN 40 MG DAILY: 21 % SECONDARY OUTCOMES Bleeding of any severity % Major or clinically relevant non major bleeding % Serious adverse events % Conclusion: Postoperative factor Xla inhibition with oral milvexian in patients undergoing knee arthroplasty was effective for the prevention of venous thromboembolism and was associated with a low risk of bleeding JI Weitz et al. NEJM 2021; 385.2161-2172