The Baby-OSCAR Trial: Evaluating Ibuprofen in Preterm Infants with PDA
In 2024, the Baby-OSCAR Trial emerged as a pivotal study examining the effects of early treatment of patent ductus arteriosus (PDA) with ibuprofen in extremely preterm infants. This randomized, double-blind, placebo-controlled trial aimed to offer new insights into the management of PDA, a common condition in preterm neonates.
Objective of the Baby-OSCAR Trial
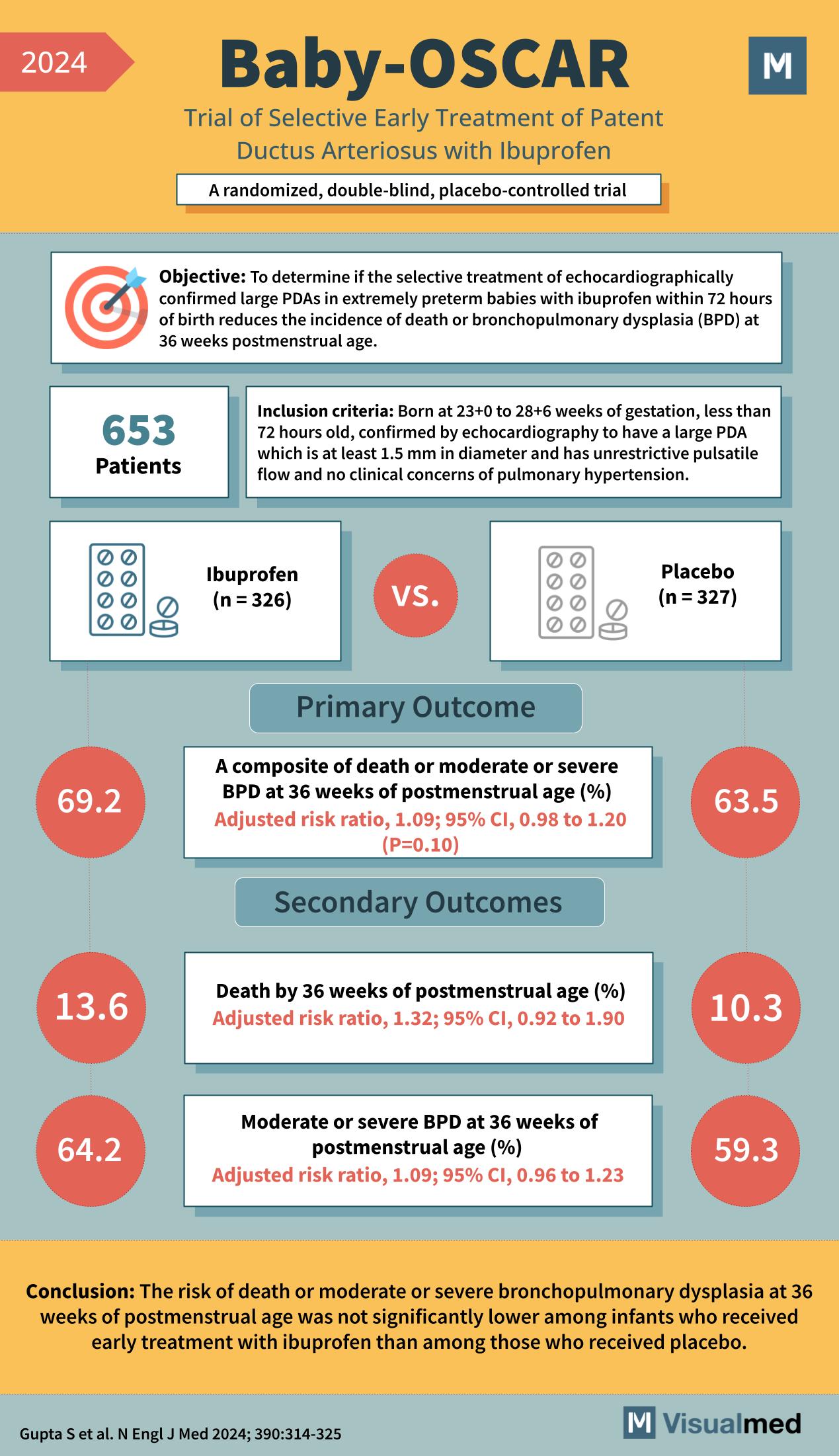
The Baby-OSCAR Trial aimed to determine if the selective early treatment of echocardiographically confirmed large PDAs with ibuprofen within 72 hours of birth could reduce the incidence of death or bronchopulmonary dysplasia (BPD) at 36 weeks postmenstrual age.
Trial Participants and Eligibility
The study included 653 extremely preterm infants born between 23+0 to 28+6 weeks of gestation. The eligibility criteria specified the infants should have a large PDA, confirmed by echocardiography, with a minimum diameter of 1.5 mm, unrestricted pulsatile flow, and no clinical signs of pulmonary hypertension.
Interventions
The infants were randomly assigned to receive either ibuprofen (326 infants) or a placebo (327 infants) as part of the treatment regime.
Findings from the Baby-OSCAR Trial
Primary Outcome
- The composite of death or moderate or severe BPD at 36 weeks of postmenstrual age was 69.2% in the ibuprofen group compared to 63.5% in the placebo group. The adjusted risk ratio was 1.09, which was not statistically significant (P=0.10).
Secondary Outcomes
- The rate of death by 36 weeks of postmenstrual age was 13.6% in the ibuprofen group versus 10.3% in the placebo group, with an adjusted risk ratio of 1.32 (P=0.10).
- Moderate or severe BPD at 36 weeks of postmenstrual age occurred in 64.2% of the ibuprofen group compared to 59.3% in the placebo group, with an adjusted risk ratio of 1.09 (P=0.10).
Conclusions and Implications
The Baby-OSCAR Trial concluded that the risk of death or moderate or severe BPD at 36 weeks postmenstrual age was not significantly lower among infants who received early treatment with ibuprofen than those who received a placebo. These findings suggest that early treatment with ibuprofen does not substantially alter the course or outcome of PDA in extremely preterm infants.