The CAPItello-291 Trial: A New Horizon in Breast Cancer Treatment
The CAPItello-291 Trial, a Phase III double-blind randomized trial conducted in 2023, has significantly contributed to the evolving landscape of breast cancer treatment, particularly for hormone receptor-positive advanced cases.
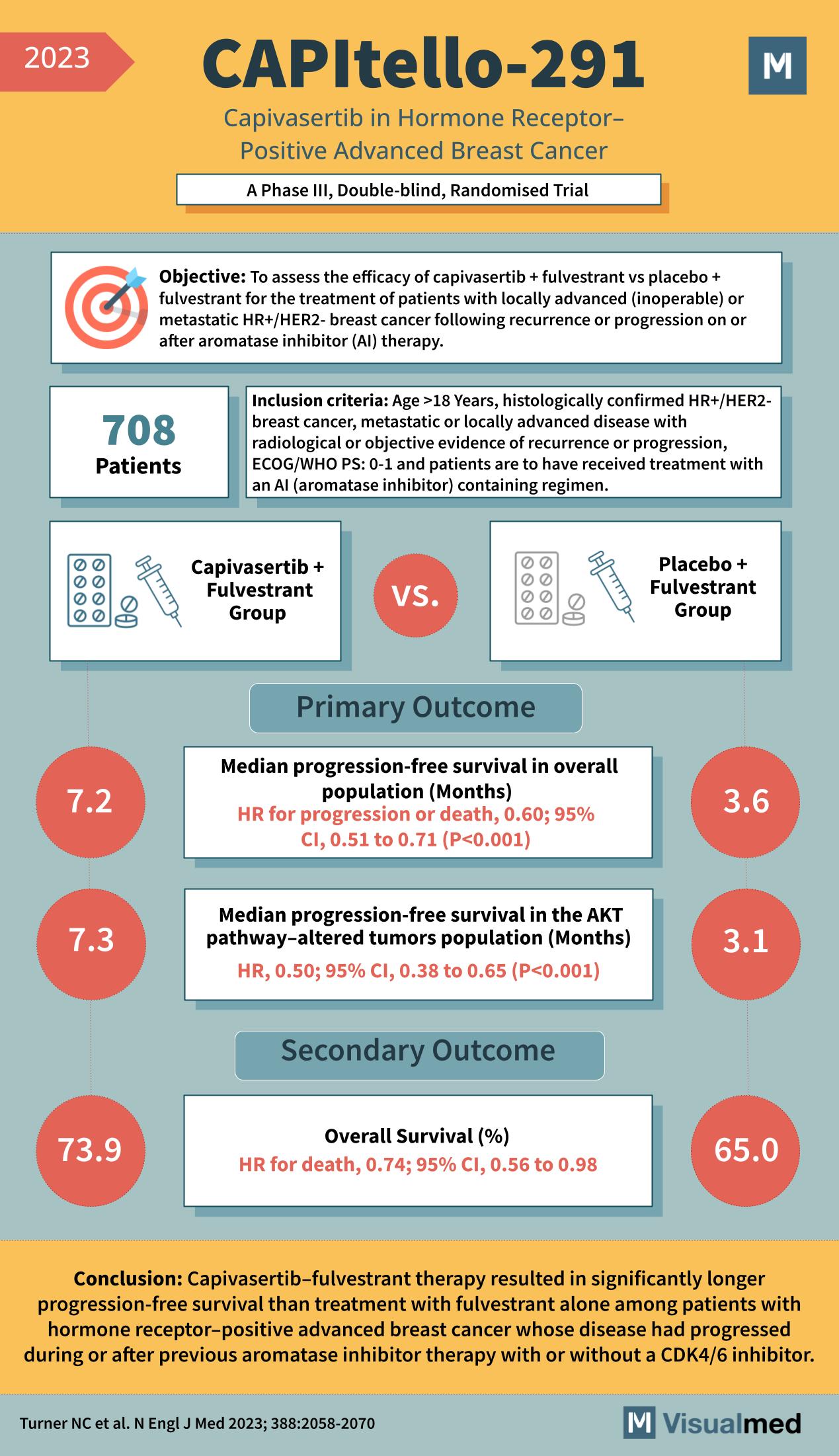
Objective of the CAPItello-291 Trial
The trial’s aim was to determine the effectiveness of the combination of capivasertib and fulvestrant versus placebo plus fulvestrant in patients with locally advanced or metastatic hormone receptor-positive, HER2-negative breast cancer that had progressed on aromatase inhibitor therapy.
Study Demographics and Inclusion Criteria
708 patients were enrolled, all over 18 years of age, with histologically confirmed HR+/HER2- breast cancer, either metastatic or locally advanced. All participants had shown disease progression during or after an aromatase inhibitor-containing regimen.
Treatment Groups
Participants were divided into two groups: one received capivasertib plus fulvestrant, while the other was treated with a placebo in addition to fulvestrant.
Results of the CAPItello-291 Trial
Primary Outcomes
- Median progression-free survival in the overall population was notably longer in the capivasertib plus fulvestrant group (7.2 months) compared to the placebo group (3.6 months), with a hazard ratio (HR) for progression or death of 0.60.
- In the AKT pathway-altered tumors population, the capivasertib group also demonstrated a higher median progression-free survival of 7.3 months versus 3.1 months in the placebo group, with an HR of 0.50.
Secondary Outcome
- Overall survival was significantly improved in the capivasertib plus fulvestrant group, with 73.9% compared to 65.0% in the placebo group, and an HR for death of 0.74.
Conclusions and Clinical Relevance
The CAPItello-291 Trial concluded that the combination therapy of capivasertib and fulvestrant resulted in a significantly longer progression-free survival compared to fulvestrant alone in patients with hormone receptor-positive advanced breast cancer. This promising outcome marks capivasertib-fulvestrant therapy as a potent option for patients who have previously experienced disease progression on or after aromatase inhibitor therapy.