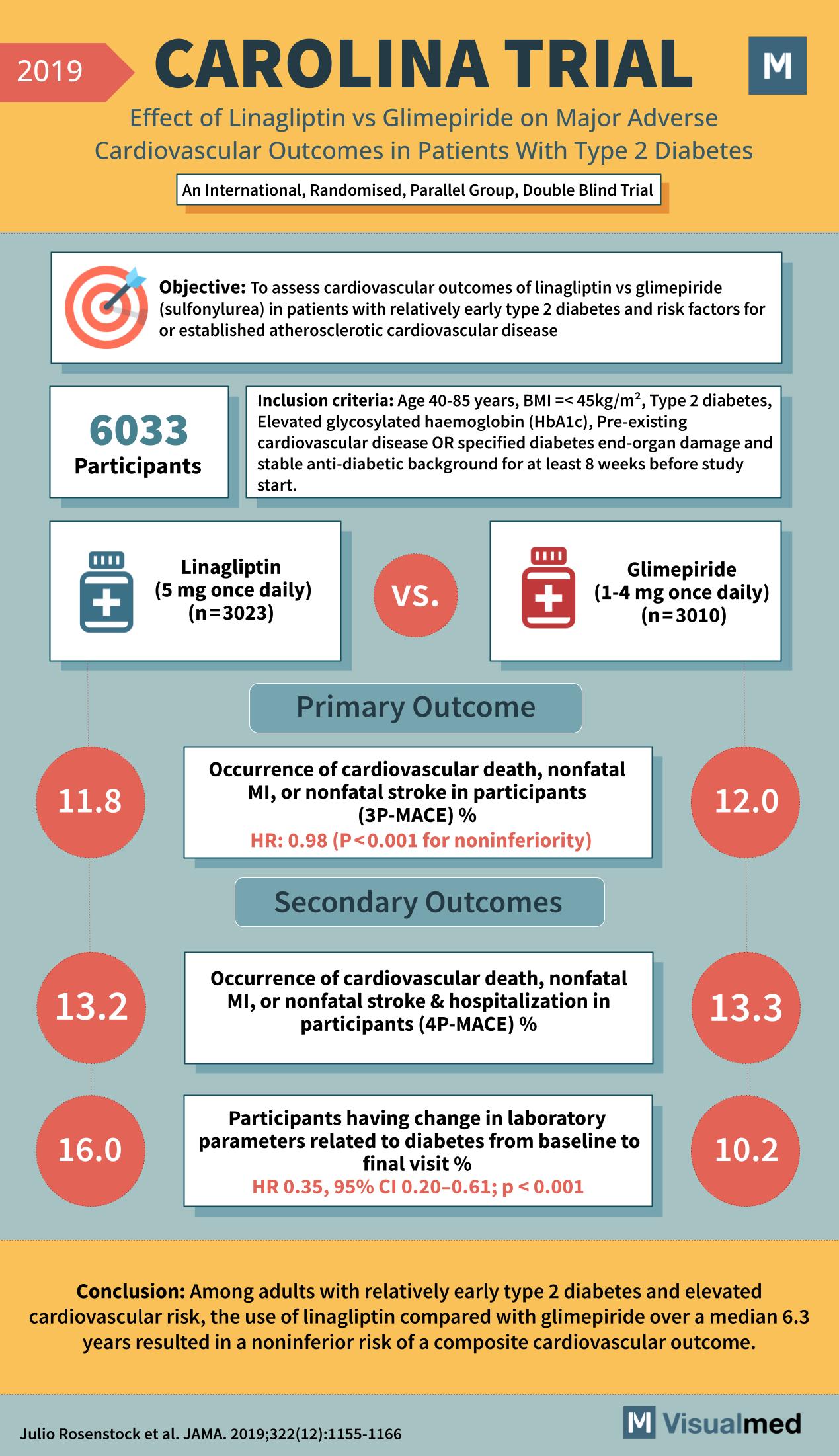
Year: 2019 Title: CAROLINA TRIAL Subtitle: Effect of Linagliptin vs Glimepiride on Major Adverse Cardiovascular Outcomes in Patients With Type 2 Diabetes Type of Trial: An International, Randomised, Parallel Group, Double Blind Trial
Objective: To assess cardiovascular outcomes of linagliptin vs glimepiride (sulfonylurea) in patients with relatively early type 2 diabetes and risk factors for or established atherosclerotic cardiovascular disease.
Participants: 6033
Inclusion Criteria:
- Age 40-85 years
- BMI ≤45kg/m^2
- Type 2 diabetes
- Elevated glycosylated haemoglobin (HbA1c)
- Pre-existing cardiovascular disease OR specified diabetes end-organ damage
- Stable anti-diabetic background for at least 8 weeks before study start
Groups:
- Linagliptin (5 mg once daily) (n=3023)
- Glimepiride (1-4 mg once daily) (n=3010)
Primary Outcome:
- Occurrence of cardiovascular death, nonfatal MI, or nonfatal stroke in participants (3P-MACE) %
- Linagliptin Group: 11.8%
- Glimepiride Group: 12.0%
- HR, 0.98 (P<0.001 for noninferiority)
Secondary Outcomes:
- Occurrence of cardiovascular death, nonfatal MI, or nonfatal stroke & hospitalization in participants (4P-MACE) %
- Linagliptin Group: 13.2%
- Glimepiride Group: 13.3%
- Participants having change in laboratory parameters related to diabetes from baseline to final visit %
- Linagliptin Group: 16.0%
- Glimepiride Group: 10.2%
- HR, 0.35; 95% CI 0.20-0.61; p < 0.001
Conclusion: Among adults with relatively early type 2 diabetes and elevated cardiovascular risk, the use of linagliptin compared with glimepiride over a median 6.3 years resulted in a noninferior risk of a composite cardiovascular outcome.
Reference: Julio Rosenstock et al. JAMA. 2019;322(12):1155-1166