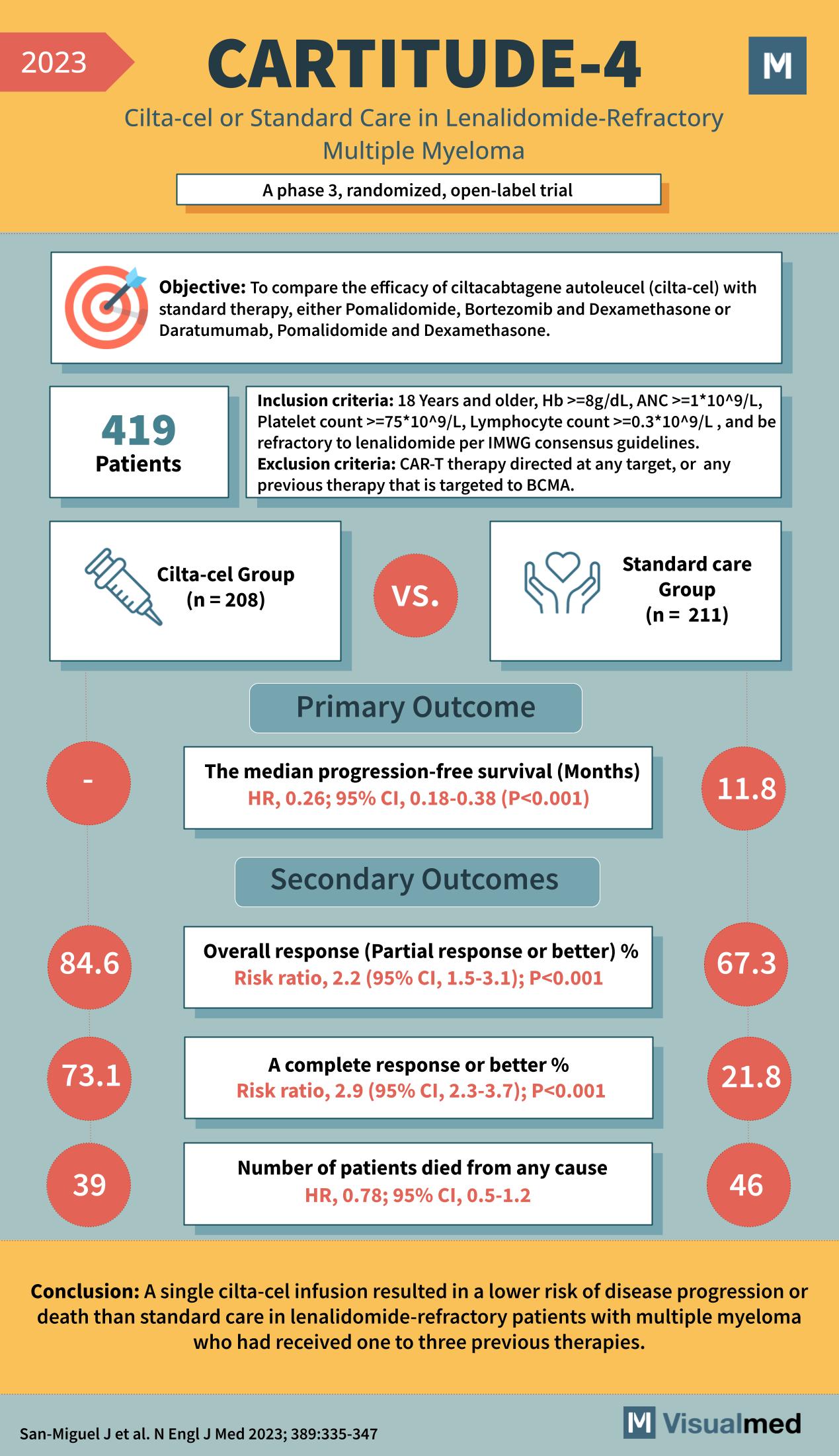
The CARTITUDE-4 Trial: Groundbreaking Treatment for Multiple Myeloma
The CARTITUDE-4 Trial, a pivotal study published in 2023, represents a significant advancement in the treatment of multiple myeloma, particularly for patients with lenalidomide-refractory disease. This Phase 3 randomized, open-label trial assessed the efficacy of a novel CAR-T therapy, cilta-cel, against standard care.
Objective of the CARTITUDE-4 Trial
The trial aimed to compare the efficacy of cilta-cel therapy to standard treatments, including Pomalidomide, Bortezomib, and Dexamethasone or Daratumumab, in patients with lenalidomide-refractory multiple myeloma.
Patient Demographics and Inclusion Criteria
The study enrolled 419 patients, all 18 years or older, with a hemoglobin level of ≥8 g/dL, and specific blood cell counts within the range required for CAR-T therapy eligibility. All participants had previously undergone one to three different therapies and were refractory to lenalidomide treatment.
Treatment Comparison
Participants were split into two groups: the cilta-cel group with 208 patients and the standard care group with 211 patients.
Trial Findings
Primary Outcome
- Median progression-free survival was not yet reached in the cilta-cel group at the time of reporting, indicating a significantly improved outcome compared to 11.8 months in the standard care group, with a hazard ratio (HR) of 0.26.
Secondary Outcomes
- The overall response rate (partial response or better) was 84.6% in the cilta-cel group, which was substantially higher than 67.3% in the standard care group, with a risk ratio of 2.2.
- A complete response or better was achieved by 73.1% of patients in the cilta-cel group versus 21.8% in the standard care group, with a risk ratio of 2.9.
- The number of patients who died from any cause was lower in the cilta-cel group (39) compared to the standard care group (46), with an HR for death of 0.78.
Conclusions and Clinical Implications
The CARTITUDE-4 Trial concluded that a single infusion of cilta-cel resulted in a lower risk of disease progression or death than standard care in lenalidomide-refractory patients with multiple myeloma. These results underscore cilta-cel as a potentially transformative treatment for patients with multiple myeloma, offering hope for those who have exhausted other therapeutic options.