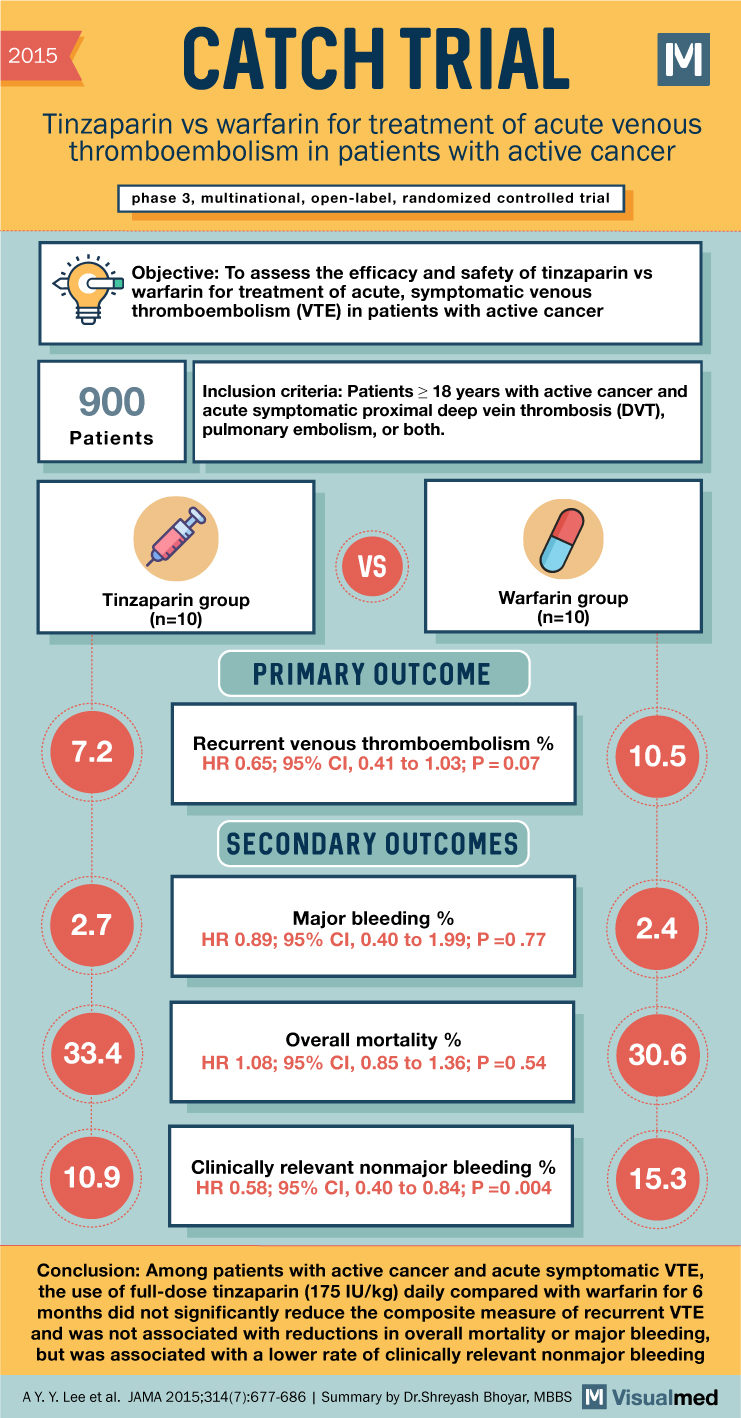
2015 CATCH TRIAL Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer phase 3, multinational, open-label, randomized controlled trial Objective: To assess the efficacy and safety of tinzaparin vs warfarin for treatment of acute, symptomatic venous thromboembolism (VTE) in patients with active cancer 900 Patients Inclusion criteria: Patients ≥ 18 years with active cancer and acute symptomatic proximal deep vein thrombosis (DVT), pulmonary embolism, or both. 0-T VS Tinzaparin group (n=10) Warfarin group (n=10) 7.2 PRIMARY OUTCOME Recurrent venous thromboembolism % HR 0.65; 95% CI, 0.41 to 1.03; P = 0.07 SECONDARY OUTCOMES 10.5 2.7 Major bleeding % 2.4 HR 0.89; 95% CI, 0.40 to 1.99; P=0.77 Overall mortality% 33.4 30.6 HR 1.08; 95% CI, 0.85 to 1.36; P=0.54 10.9 Clinically relevant nonmajor bleeding % HR 0.58; 95% CI, 0.40 to 0.84; P=0.004 15.3 Conclusion: Among patients with active cancer and acute symptomatic VTE, the use of full-dose tinzaparin (175 IU/kg) daily compared with warfarin for 6 months did not significantly reduce the composite measure of recurrent VTE and was not associated with reductions in overall mortality or major bleeding, but was associated with a lower rate of clinically relevant nonmajor bleeding AY. Y. Lee et al. JAMA 2015;314(7):677-686