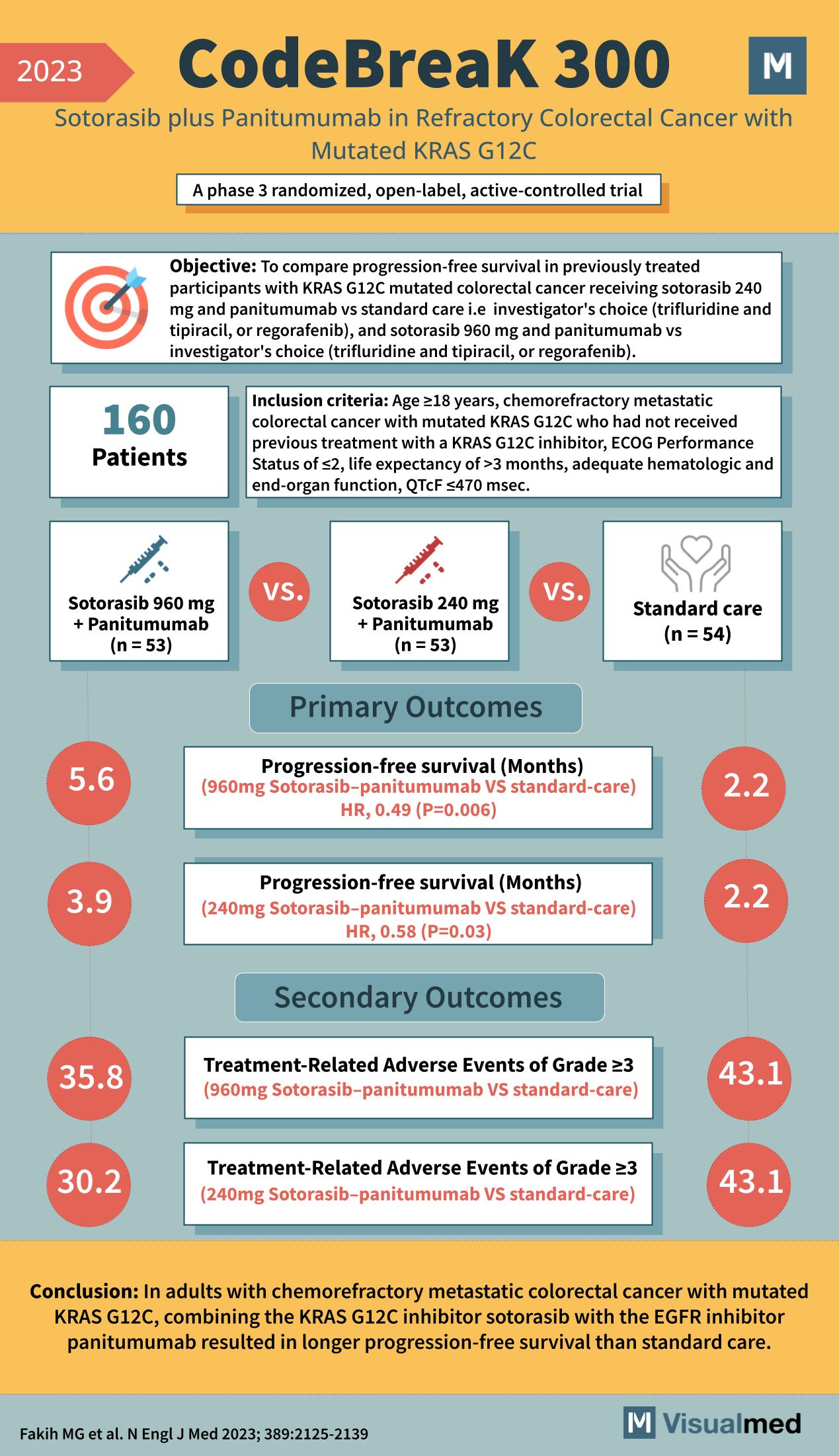
Year: 2023 Title: CodeBreaK 300 Subtitle: Sotorasib plus Panitumumab in Refractory Colorectal Cancer with Mutated KRAS G12C Type of Trial: A phase 3 randomized, open-label, active-controlled trial
Objective: To compare progression-free survival in previously treated participants with KRAS G12C mutated colorectal cancer receiving sotorasib plus panitumumab vs standard care, i.e., investigator’s choice (trifluridine and tipiracil, or regorafenib), and sotorasib 960 mg and panitumumab vs investigator’s choice (trifluridine and tipiracil, or regorafenib).
Participants: 160
Inclusion Criteria:
- Age ≥18 years
- Chemorefractory metastatic colorectal cancer with mutated KRAS G12C who had not received previous treatment with a KRAS G12C inhibitor
- ECOG Performance Status of ≤2
- Life expectancy of >3 months
- Adequate hematologic and end-organ function
- QTcF ≤470 msec.
Groups:
- Sotorasib 960 mg + Panitumumab (n = 53)
- Sotorasib 240 mg + Panitumumab (n = 53)
- Standard care (n = 54)
Primary Outcomes:
- Progression-free survival (Months) for 960mg Sotorasib-panitumumab vs standard-care
- HR, 0.49 (P=0.006)
- Sotorasib-Panitumumab Group: 5.6 months
- Standard Care Group: 2.2 months
- Progression-free survival (Months) for 240mg Sotorasib-panitumumab vs standard-care
- HR, 0.58 (P=0.03)
- Sotorasib-Panitumumab Group: 3.9 months
- Standard Care Group: 2.2 months
Secondary Outcomes:
- Treatment-Related Adverse Events of Grade ≥3 for 960mg Sotorasib-panitumumab vs standard-care
- Sotorasib-Panitumumab Group: 35.8%
- Standard Care Group: 43.1%
- Treatment-Related Adverse Events of Grade ≥3 for 240mg Sotorasib-panitumumab vs standard-care
- Sotorasib-Panitumumab Group: 30.2%
- Standard Care Group: 43.1%
Conclusion: In adults with chemorefractory metastatic colorectal cancer with mutated KRAS G12C, combining the KRAS G12C inhibitor sotorasib with the EGFR inhibitor panitumumab resulted in longer progression-free survival than standard care.
Reference: Fakih MG et al. N Engl J Med 2023; 389:2125-2139