CONVINCE Trial Summary
The abstract discusses the results of the CONVINCE trial, a randomized, controlled study comparing high-dose hemodiafiltration (HDF) with conventional high-flux hemodialysis (HD) in patients with end-stage kidney disease (ESKD). The trial aimed to determine whether high-dose HDF reduces the risk of death in ESKD patients compared to high-flux HD.
Previous studies had suggested potential benefits of high-dose HDF over standard hemodialysis, but more data were needed due to limitations in existing research. The CONVINCE trial was designed as an investigator-initiated, international, multicenter, prospective study to address this gap.
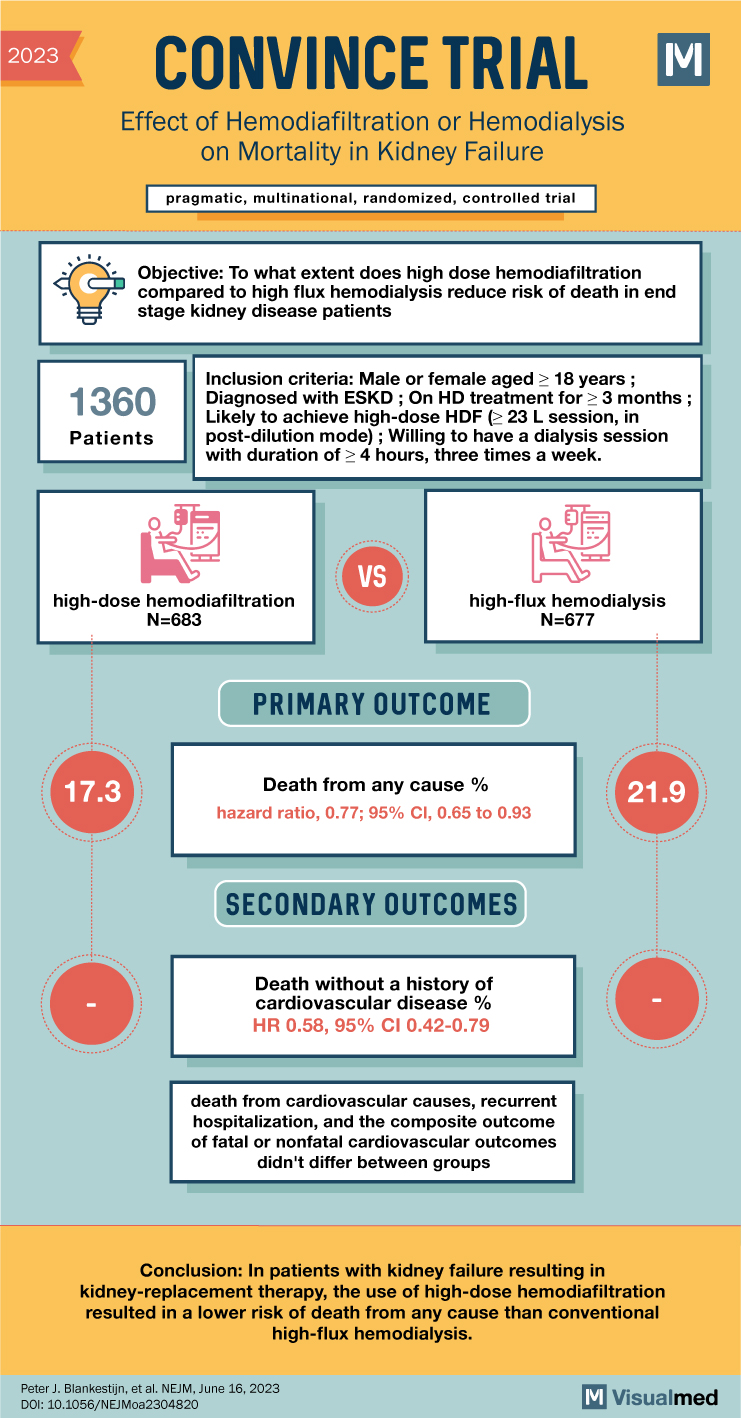
The inclusion criteria for participants required them to be diagnosed with ESKD, receiving HD treatment for at least three months, and likely to achieve high-dose HDF as per the protocol. They also needed to be willing to undergo dialysis sessions of at least four hours, three times a week, and demonstrate an understanding of the study procedures.
Certain exclusion criteria were established, such as severe non-compliance with dialysis procedures, a life expectancy of less than three months, recent HDF treatment, and anticipated living donor kidney transplantation within six months. Other exclusion factors included diseases or medical conditions that could interfere with treatment or increase the risk of complications, participation in conflicting studies, and unavailability for study visits lasting three months or more.
The trial utilized a pragmatic approach and included patients who had been on high-flux HD for at least three months. Participants were randomized into two groups: one receiving high-dose HDF and the other continuing with high-flux HD. The primary outcome assessed was death from any cause, with key secondary outcomes including cause-specific death, composite cardiovascular events, kidney transplantation, and hospitalizations due to all-cause or infection-related reasons.
A total of 1,360 patients were randomized, with 683 in the high-dose HDF group and 677 in the high-flux HD group. The median follow-up period was 30 months. During the trial, the average convection volume for the HDF group was 25.3 liters per session. The analysis revealed that death from any cause occurred in 118 patients (17.3%) in the HDF group and 148 patients (21.9%) in the HD group. The hazard ratio for death was 0.77, indicating a lower risk of death in the high-dose HDF group compared to conventional high-flux HD.
In conclusion, the CONVINCE trial demonstrated that high-dose hemodiafiltration was associated with a reduced risk of death from any cause in patients with kidney failure requiring kidney-replacement therapy. This finding suggests that high-dose HDF may be a more effective treatment option compared to conventional high-flux HD. The results of this trial provide additional data supporting the potential benefits of high-dose HDF in patients with end-stage kidney disease, and further research in this area may be warranted.