The COSMIC-313 Trial: A New Frontier in Renal-Cell Carcinoma Treatment
In the landscape of oncology, renal-cell carcinoma stands as a formidable challenge, often presenting at advanced stages and with a dire need for effective treatments. Enter the COSMIC-313 Trial, a phase 3, double-blind, randomized controlled trial that has shed light on a promising new combination therapy. This trial aimed to assess the efficacy and safety of combining cabozantinib with nivolumab and ipilimumab in patients with previously untreated advanced renal-cell carcinoma.
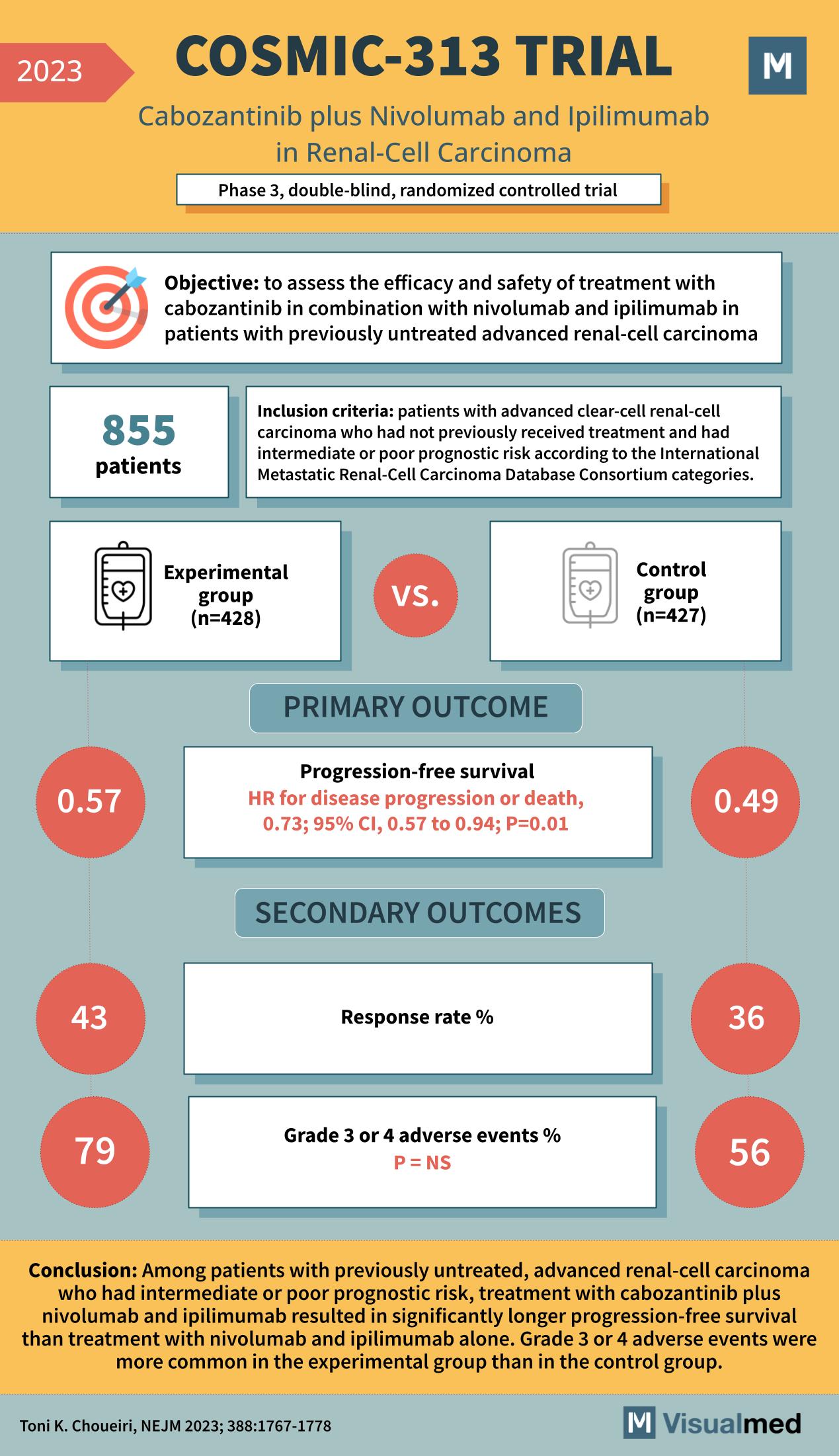
Objective and Inclusion Criteria
The trial enrolled 855 patients who met stringent inclusion criteria: those with advanced clear-cell renal-cell carcinoma who had not previously received treatment and were identified as having intermediate or poor prognostic risk according to the International Metastatic Renal-Cell Carcinoma Database Consortium categories.
Treatment Arms
Participants were divided into two groups. The experimental group, consisting of 428 patients, received a combination treatment of cabozantinib, nivolumab, and ipilimumab. The control group, with 427 patients, was treated with nivolumab and ipilimumab alone, standard care for this patient population.
Primary Outcome: Progression-Free Survival
The primary outcome of the COSMIC-313 Trial was progression-free survival, defined as the length of time during and after the treatment that a patient lives with the disease without it getting worse. The trial reported a hazard ratio for disease progression or death of 0.73; this indicates a 27% reduction in the risk of disease progression or death in the experimental group compared to the control group, with a 95% confidence interval of 0.57 to 0.94 and a p-value of 0.01, signifying statistical significance.
Secondary Outcomes
The secondary outcomes included response rate and the incidence of grade 3 or 4 adverse events. The experimental group saw a response rate of 43% versus 36% in the control group. However, there was a higher incidence of grade 3 or 4 adverse events in the experimental group, at 79%, compared to 56% in the control group, although the difference was not statistically significant (p=NS).
Conclusion and Impact
The conclusion drawn from the COSMIC-313 Trial was that the combination of cabozantinib with nivolumab and ipilimumab led to a significantly longer progression-free survival than the standard treatment regimen. However, it was also observed that higher-grade adverse events were more common in the experimental group.
The implications of the COSMIC-313 Trial are far-reaching. For patients with previously untreated advanced renal-cell carcinoma, especially those with an intermediate or poor prognosis, this combination therapy represents a new potential line of treatment. While the increase in adverse events is a concern, the benefits of longer progression-free