Pioneering Cardiac Care: Results from the DCD HEART Trial
The DCD HEART Trial has broken new ground in the field of heart transplantation, examining the outcomes with donor hearts after circulatory death—a method that could potentially increase the donor pool significantly. The 2023 unblinded, randomized, non-inferiority trial has produced vital data that may change the future of cardiac transplantations.
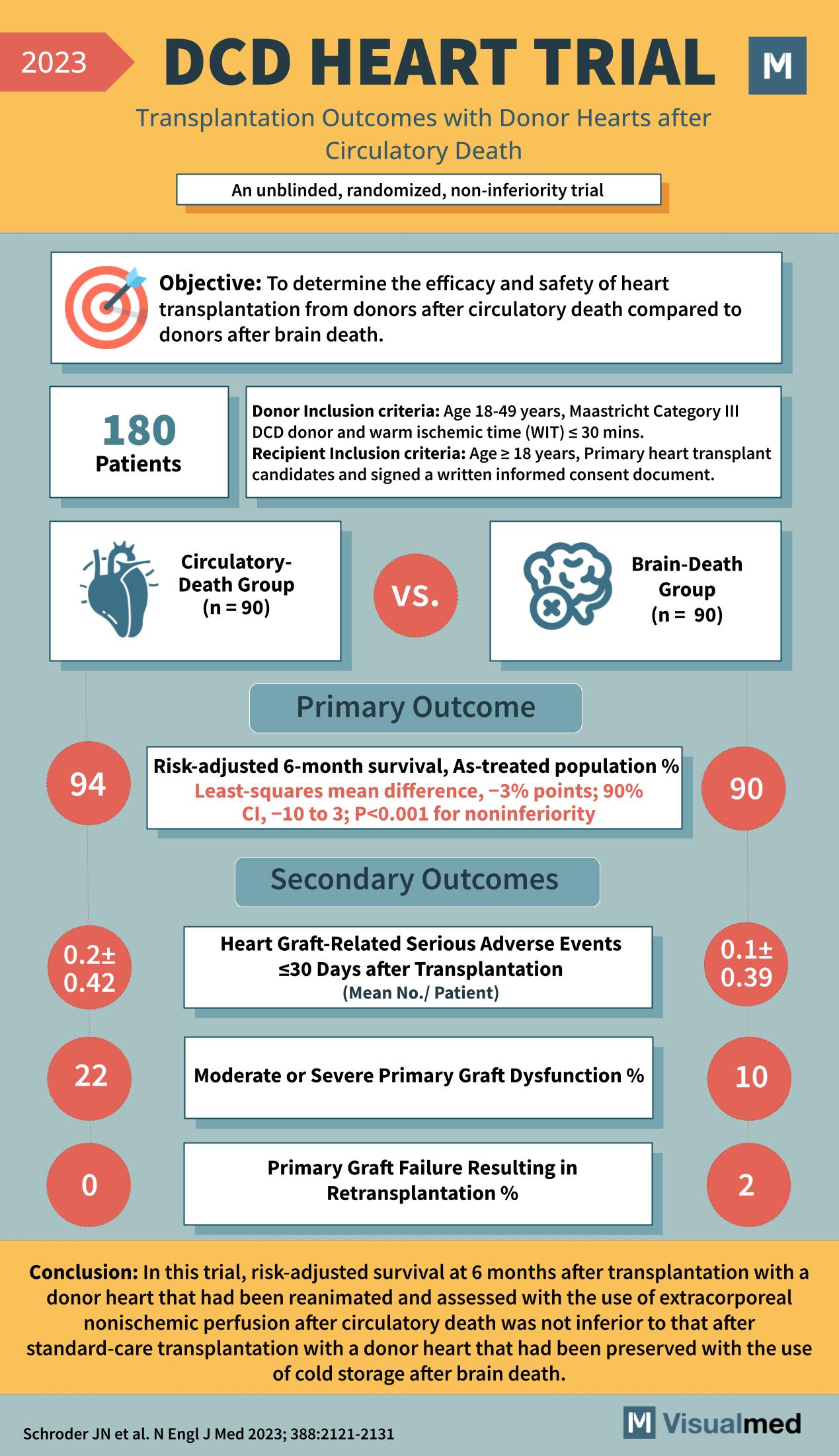
Objective of the DCD HEART Trial
The main goal of the DCD HEART Trial was to assess the efficacy and safety of heart transplantation from donors after circulatory death compared to donors after brain death. This ambitious trial aimed to provide a robust alternative to the limited donor heart options available.
Trial Participants and Criteria
With 180 patients enrolled, the trial set strict inclusion criteria for both donors and recipients. Donors aged 18-49 years and classified as Maastricht Category III DCD with warm ischemic time (WIT) ≤ 30 minutes were selected. Recipients included individuals aged ≥18 years who were primary heart transplant candidates and had provided informed consent.
Comparing Circulatory-Death and Brain-Death Donor Outcomes
Participants were divided into two groups: 90 received hearts from the circulatory-death group, and another 90 from the brain-death group.
Clinical Findings of the DCD HEART Trial
Primary Outcome
The trial’s primary outcome was risk-adjusted 6-month survival. In the circulatory-death group, the survival percentage was 94% with a least-squares mean difference of -3% points compared to the brain-death group, proving non-inferiority with a significant p-value (P<0.001).
Secondary Outcomes
Secondary outcomes were critical in understanding the safety and effectiveness of the transplantation process. Heart graft-related serious adverse events within 30 days post-transplantation averaged 0.2 per patient in the circulatory-death group, contrasting with 0.1 in the brain-death group. Moderate or severe primary graft dysfunction was reported at 22% in the circulatory-death group and 10% in the brain-death group. Primary graft failure resulting in retransplantation was reported at 0% for the circulatory-death group and 2% for the brain-death group.
Conclusions from the DCD HEART Trial
The trial concluded that the use of a donor heart from circulatory death, reanimated and assessed with extracorporeal nonischemic perfusion, was not inferior to that of a standard-care transplantation with a donor heart from brain death. This opens up a new horizon for heart transplantations, potentially reducing wait times and saving more lives.
Impact on Heart Transplantation Practices
The DCD HEART Trial results signify a leap forward in donor heart utilization, providing a beacon of hope for those on transplant waitlists. The trial demonstrates the viability of using hearts from circulatory-death donors, ensuring that more patients can receive the life-saving procedures they urgently need.