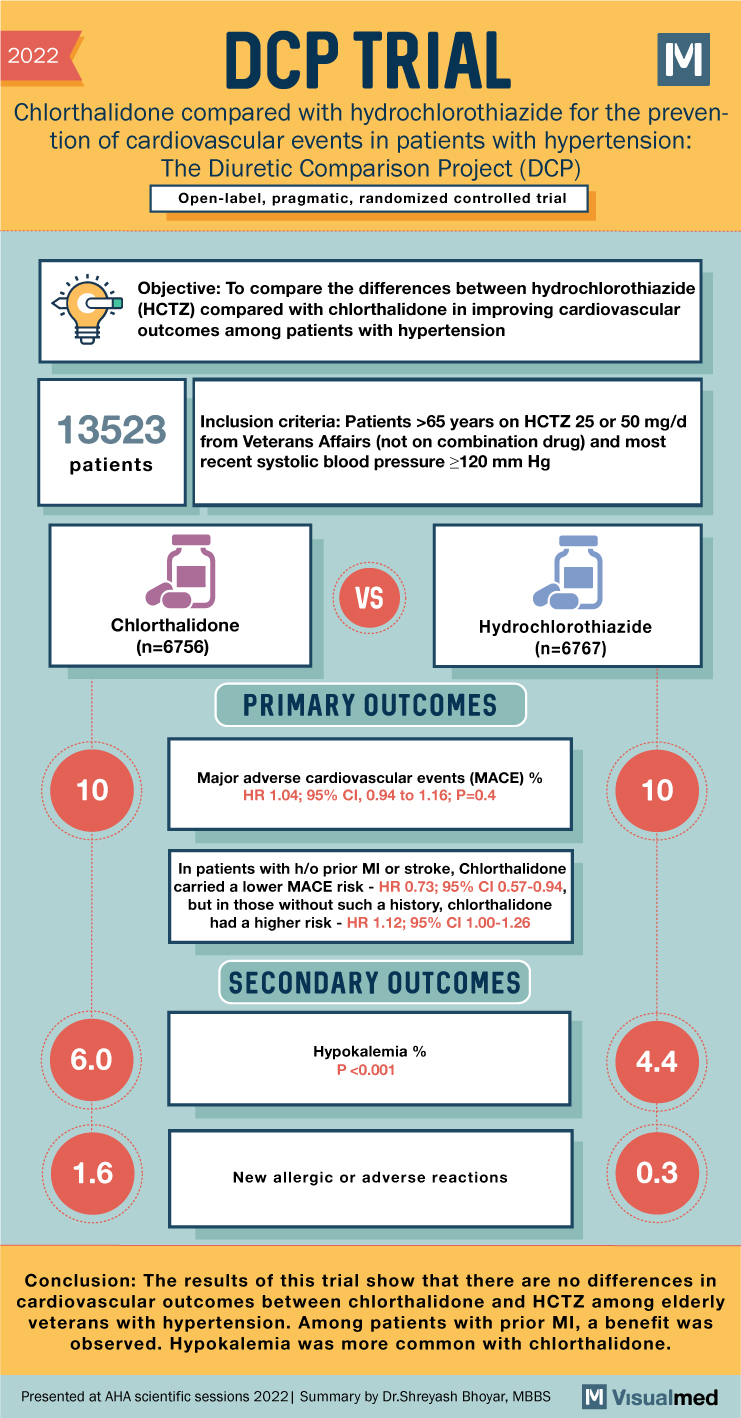
2022 DCP TRIAL Chlorthalidone compared with hydrochlorothiazide for the preven- tion of cardiovascular events in patients with hypertension: The Diuretic Comparison Project (DCP) Open-label, pragmatic, randomized controlled trial Objective: To compare the differences between hydrochlorothiazide (HCTZ) compared with chlorthalidone in improving cardiovascular outcomes among patients with hypertension 13523 patients Inclusion criteria: Patients >65 years on HCTZ 25 or 50 mg/d from Veterans Affairs (not on combination drug) and most recent systolic blood pressure ≥120 mm Hg Chlorthalidone 10 10 (n=6756) 6.0 VS Hydrochlorothiazide (n=6767) PRIMARY OUTCOMES Major adverse cardiovascular events (MACE) % HR 1.04; 95% CI, 0.94 to 1.16; P=0.4 In patients with h/o prior MI or stroke, Chlorthalidone carried a lower MACE risk – HR 0.73; 95% CI 0.57-0.94, but in those without such a history, chlorthalidone had a higher risk – HR 1.12; 95% CI 1.00-1.26 SECONDARY OUTCOMES Hypokalemia % P <0.001 100 10 4.4 1.6 New allergic or adverse reactions 0.3 Conclusion: The results of this trial show that there are no differences in cardiovascular outcomes between chlorthalidone and HCTZ among elderly veterans with hypertension. Among patients with prior MI, a benefit was observed. Hypokalemia was more common with chlorthalidone. Presented at AHA scientific sessions 2022