Title: ELATIVE Trial: A Landmark Study on Elafibranor’s Efficacy in Primary Biliary Cholangitis
Introduction:
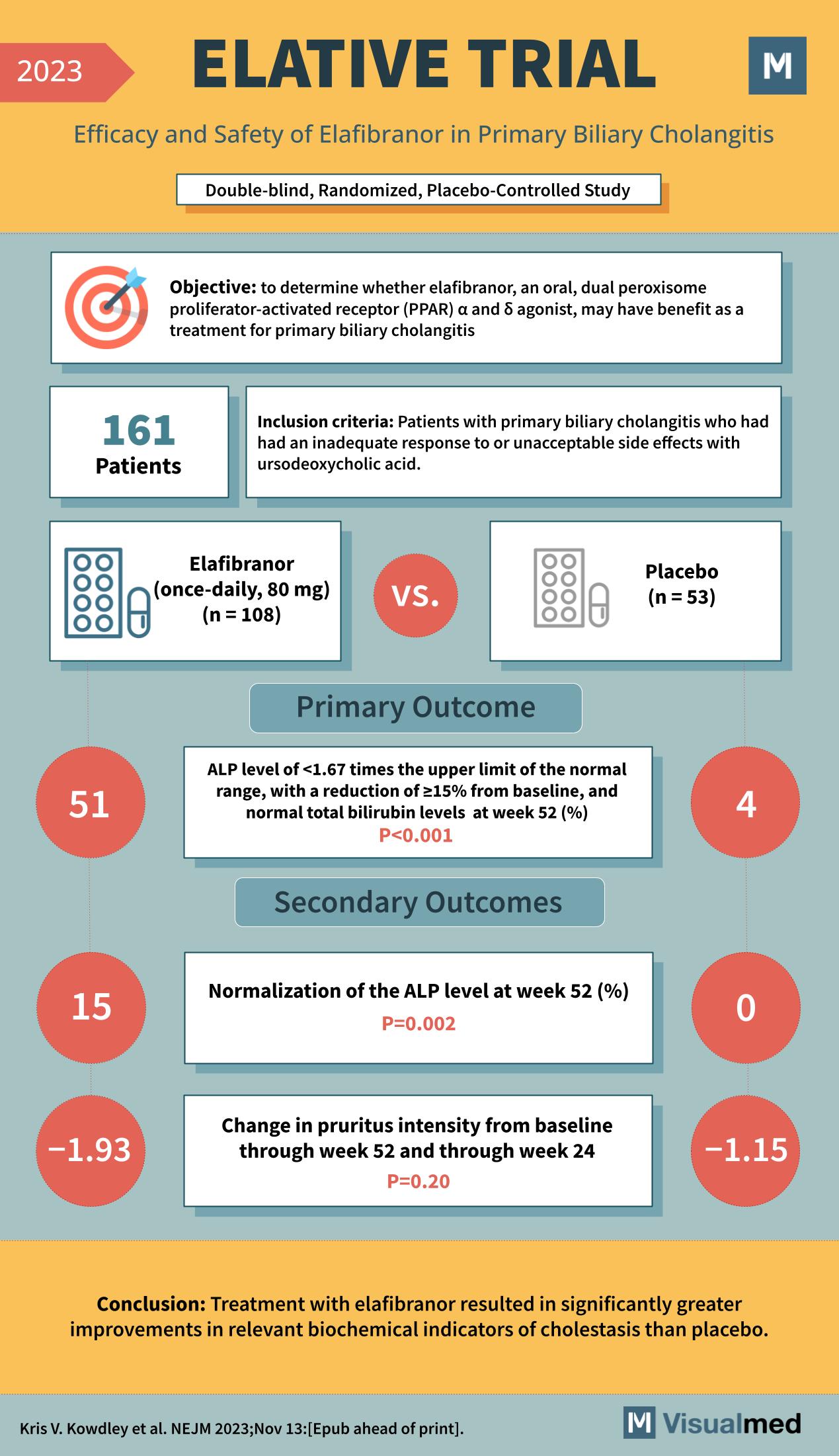
The medical community has taken a significant step forward with the recent ELATIVE Trial, a study that rigorously evaluates the efficacy and safety of Elafibranor in patients with Primary Biliary Cholangitis (PBC). This double-blind, randomized, placebo-controlled study offers new insights into the treatment of a condition that has challenged patients and healthcare providers alike.
Objective of the ELATIVE Trial:
Primary Biliary Cholangitis is a chronic liver disease characterized by the gradual destruction of bile ducts within the liver. The ELATIVE Trial was designed with a clear objective: to determine if Elafibranor, an oral medication acting on peroxisome proliferator-activated receptors, could be a beneficial treatment for patients with PBC who do not respond well to the current standard of care, ursodeoxycholic acid.
Methodology:
The trial enrolled 161 patients who met the inclusion criteria of having PBC with an inadequate response to ursodeoxycholic acid. Participants were divided into two groups: one receiving Elafibranor (108 patients) and the other receiving a placebo (53 patients). The primary endpoint was to measure the levels of alkaline phosphatase (ALP) and total bilirubin, which are critical biochemical markers for cholestasis, at the 52-week mark.
Results:
The results of the ELATIVE Trial were promising. The primary outcome showed that 51 patients in the Elafibranor group achieved the target ALP levels, which was a significant improvement compared to only 4 in the placebo group. This was a substantial finding, as a reduction in ALP levels correlates with improved prognosis in PBC patients.
Secondary Outcomes:
In addition to the primary outcome, the trial also observed secondary outcomes, such as the normalization of the ALP level at week 52 and changes in pruritus intensity. Elafibranor demonstrated a significant effect on ALP normalization, with 15% of participants reaching the desired levels as opposed to none in the placebo group. However, the change in pruritus intensity did not show a significant difference between the two groups.
Conclusion:
The ELATIVE Trial concluded that treatment with Elafibranor led to significantly greater improvements in relevant biochemical indicators of cholestasis than placebo. This groundbreaking study provides hope for better management of Primary Biliary Cholangitis and may pave the way for a new standard of care for this challenging disease.
Implications for Future Treatment:
The success of the ELATIVE Trial signifies a potential paradigm shift in the treatment of PBC. With these encouraging results, Elafibranor could soon become a part of the treatment regimen, offering relief and improved quality of life to those affected by the disease.
Final Thoughts:
The ELATIVE Trial is a testament to the relentless pursuit of better treatment options for patients with PBC. As the medical community awaits further research and long-term data, Elafibranor stands out as a beacon of hope, demonstrating the potential to change lives for the better.
Citations:
Kris V. Kowdley et al., New England Journal of Medicine 2023