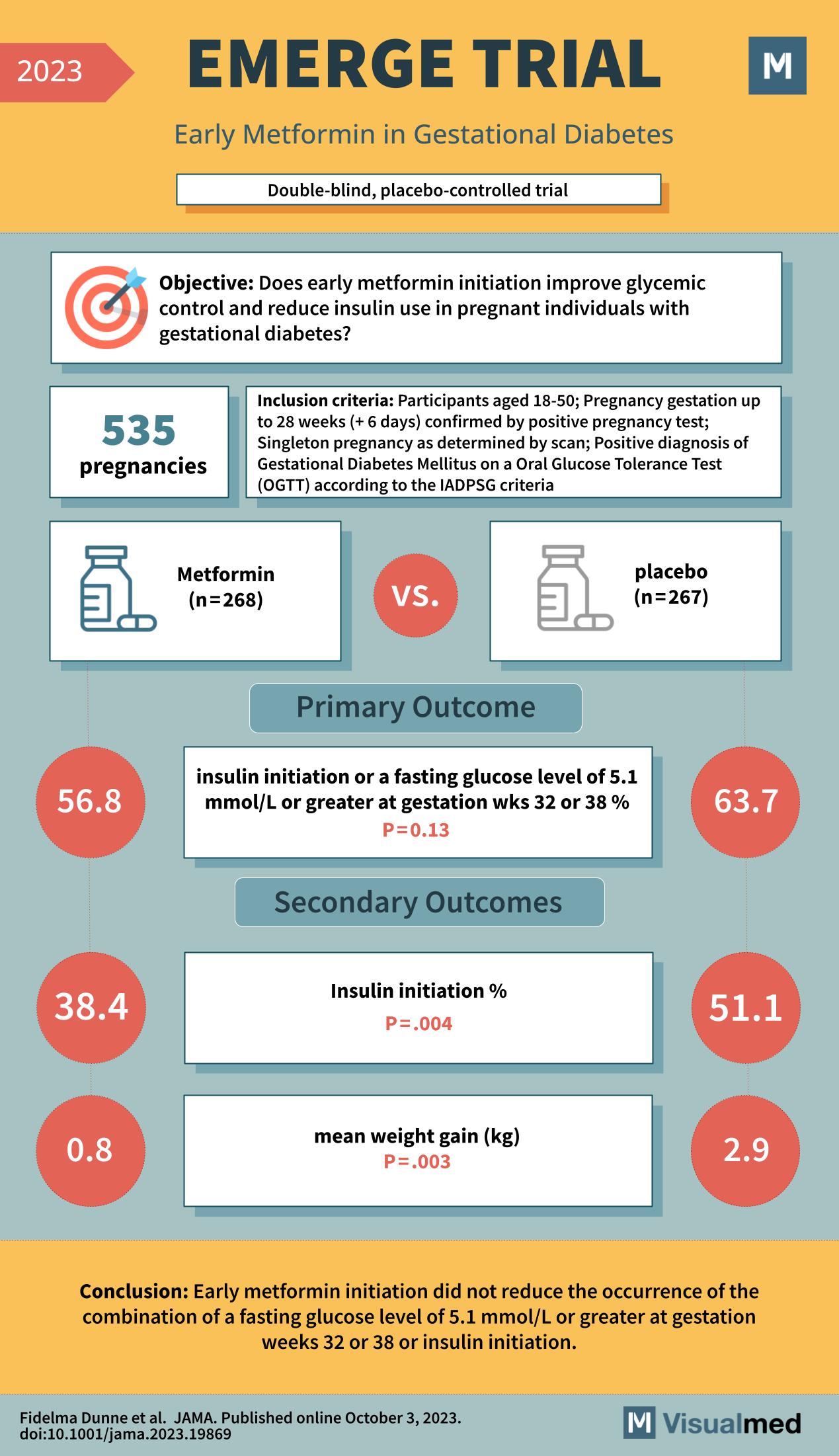
The EMERGE trial, published in JAMA in October 2023, is a pivotal study in the field of obstetric care, specifically targeting the management of gestational diabetes—a condition of elevated blood sugar that occurs during pregnancy. The trial’s goal was to determine if early initiation of metformin, a medication commonly used to treat type 2 diabetes, could improve glycemic control and reduce the need for insulin in pregnant individuals diagnosed with gestational diabetes.
This double-blind, placebo-controlled trial involved 535 pregnancies, with participants aged 18-50, and gestational age up to 28 weeks confirmed by a positive pregnancy test. To be included in the trial, the pregnancies had to be singleton as determined by scan, and there had to be a positive diagnosis of Gestational Diabetes Mellitus via an Oral Glucose Tolerance Test according to the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria.
Participants were randomized into two groups: one receiving metformin (n=268) and the other receiving a placebo (n=267). The primary outcome was the initiation of insulin therapy or a fasting glucose level of 5.1 mmol/L or greater at 32 or 38 weeks of gestation. The results showed that 56.8% of the metformin group versus 63.7% of the placebo group reached this outcome, with a p-value of 0.13, indicating no significant difference between the two groups in the primary outcome.
Secondary outcomes included insulin initiation, which was significantly lower in the metformin group at 38.4%, compared to 51.1% in the placebo group (p=0.004). Additionally, the mean weight gain was less in the metformin group, with an average of 0.8 kg, compared to 2.9 kg in the placebo group (p=0.003), indicating a potential benefit of metformin in controlling weight gain during pregnancy in women with gestational diabetes.
The EMERGE trial’s conclusion was that early metformin initiation did not reduce the occurrence of the combination of a fasting glucose level of 5.1 mmol/L or greater at gestation weeks 32 or 38 or the need for insulin initiation. However, the secondary outcomes suggest that metformin may still have benefits in terms of reducing the need for insulin and limiting weight gain during pregnancy for women with gestational diabetes.
The findings of the EMERGE trial are crucial for healthcare providers managing pregnancies complicated by gestational diabetes. While metformin may not eliminate the need for insulin, it could be considered as part of a multifaceted approach to managing the condition. The trial adds to the body of evidence that helps guide clinical decision-making and the development of guidelines for the management of gestational diabetes. It also raises further questions about the timing of pharmacological intervention and its impact on pregnancy outcomes, which could be avenues for future research.