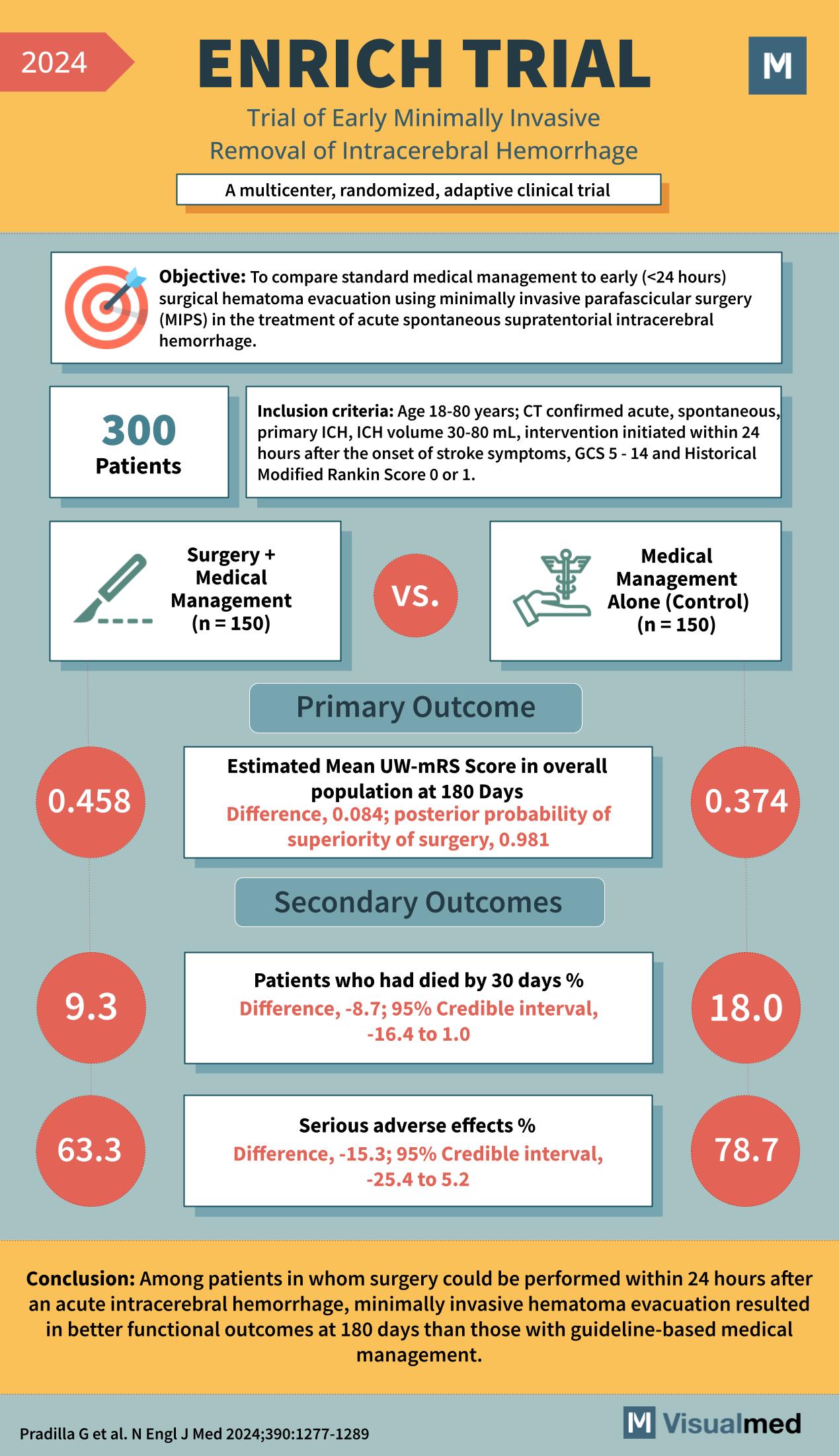
Year: 2024 Title: ENRICH TRIAL Subtitle: Trial of Early Minimally Invasive Removal of Intracerebral Hemorrhage Type of Trial: A multicenter, randomized, adaptive clinical trial
Objective: To compare standard medical management to early (<24 hours) surgical hematoma evacuation using minimally invasive parafascicular surgery (MIPS) in the treatment of acute spontaneous supratentorial intracerebral hemorrhage.
Patients: 300
Inclusion Criteria:
- Age 18-80 years
- CT confirmed acute, spontaneous, primary ICH
- ICH volume 30-80 mL
- Intervention initiated within 24 hours after the onset of stroke symptoms
- GCS 5 – 14 and Historical Modified Rankin Score 0 or 1
Groups:
- Surgery + Medical Management (n = 150)
- Medical Management Alone (Control) (n = 150)
Primary Outcome:
- Estimated Mean UW-mRS Score in the overall population at 180 Days
- Surgery + Medical Management: 0.458
- Medical Management Alone: 0.374
- Difference, 0.084; posterior probability of superiority of surgery, 0.981
Secondary Outcomes:
- Patients who had died by 30 days %
- Surgery + Medical Management: 9.3
- Medical Management Alone: 18.0
- Difference, -8.7; 95% Credible interval, -16.4 to 1.0
- Serious adverse effects %
- Surgery + Medical Management: 63.3
- Medical Management Alone: 78.7
- Difference, -15.3; 95% Credible interval, -25.4 to 5.2
Conclusion: Among patients in whom surgery could be performed within 24 hours after an acute intracerebral hemorrhage, minimally invasive hematoma evacuation resulted in better functional outcomes at 180 days than those with guideline-based medical management.
Reference: Pradilla G et al. N Engl J Med 2024;390:1277-1289