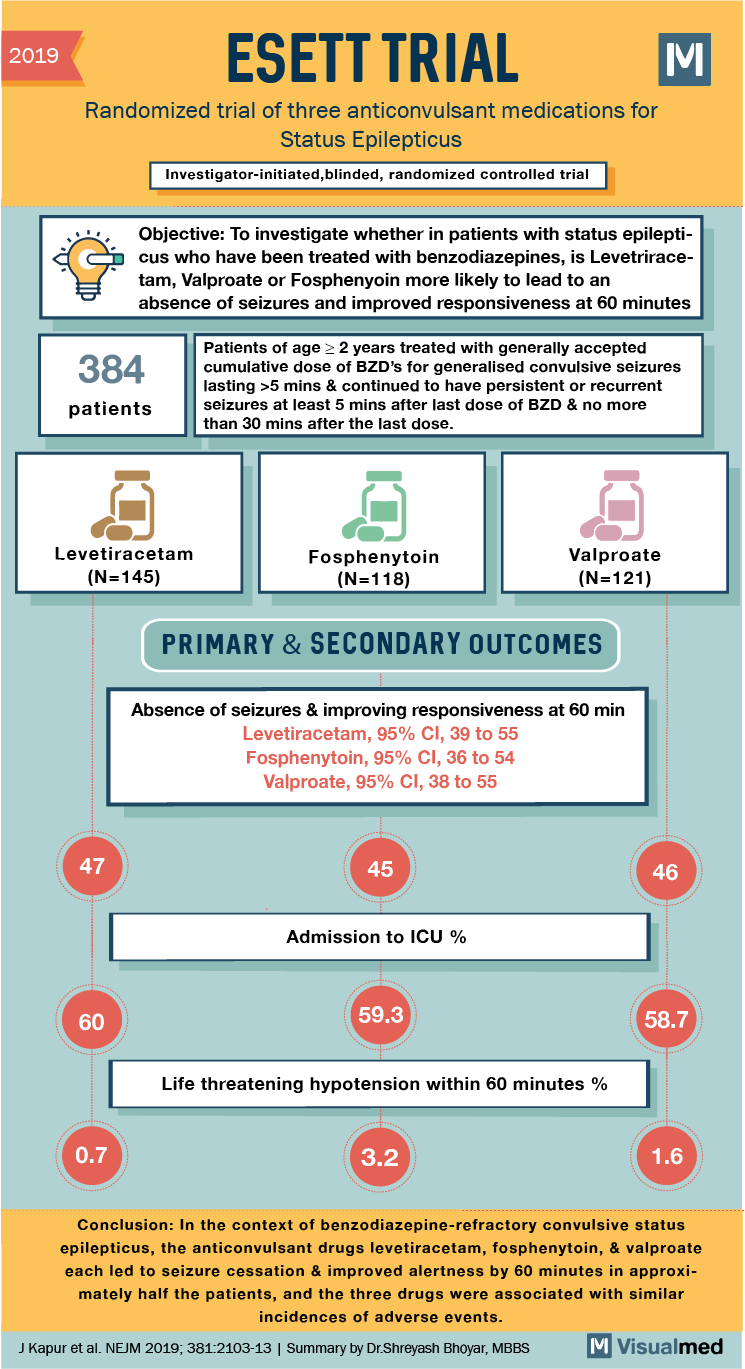
2019 ESETT TRIAL Randomized trial of three anticonvulsant medications for Status Epilepticus Investigator-initiated, blinded, randomized controlled trial Objective: To investigate whether in patients with status epilepticus who have been treated with benzodiazepines, is Levetriracetam, Valproate or Fosphenyoin more likely to lead to an absence of seizures and improved responsiveness at 60 minutes 384 patients Patients of age > 2 years treated with generally accepted cumulative dose of BZD’s for generalised convulsive seizures lasting >5 mins & continued to have persistent or recurrent seizures at least 5 mins after last dose of BZD & no more than 30 mins after the last dose. Levetiracetam (N=145) Fosphenytoin (N=118) Valproate (N=121) PRIMARY & SECONDARY OUTCOMES Absence of seizures & improving responsiveness at 60 min Levetiracetam, 95% CI, 39 to 55 Fosphenytoin, 95% CI, 36 to 54 Valproate, 95% CI, 38 to 55 45 46 Admission to ICU % 60 59.3 58.7 Life threatening hypotension within 60 minutes % 0.7 3.2 1.6 Conclusion: In the context of benzodiazepine-refractory convulsive status epilepticus, the anticonvulsant drugs levetiracetam, fosphenytoin, & valproate each led to seizure cessation & improved alertness by 60 minutes in approximately half the patients, and the three drugs were associated with similar incidences of adverse events. J Kapur et al. NEJM 2019, 381-2103-13