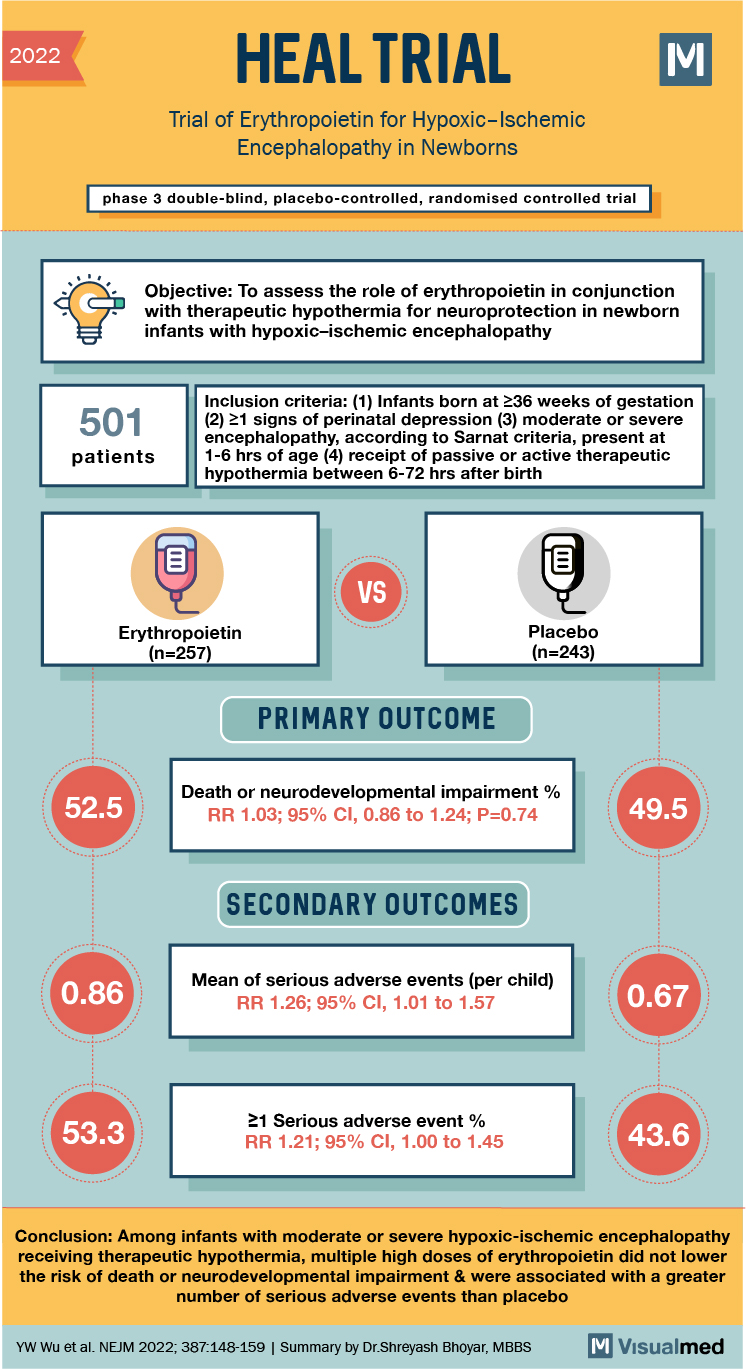
2022 HEAL TRIAL Trial of Erythropoietin for Hypoxic-Ischemic Encephalopathy in Newborns phase 3 double-blind, placebo-controlled, randomised controlled trial Objective: To assess the role of erythropoietin in conjunction with therapeutic hypothermia for neuroprotection in newborn infants with hypoxic-ischemic encephalopathy 501 Inclusion criteria: (1) Infants born at 236 weeks of gestation (2) 21 signs of perinatal depression (3) moderate or severe encephalopathy, according to Sarnat criteria, present at 1-6 hrs of age (4) receipt of passive or active therapeutic hypothermia between 6-72 hrs after birth patients VS Erythropoietin (n=257) Placebo (n=243) PRIMARY OUTCOME 52.5 Death or neurodevelopmental impairment % RR 1.03; 95% CI, 0.86 to 1.24; P=0.74 49.5 SECONDARY OUTCOMES 0.86 Mean of serious adverse events (per child) RR 1.26; 95% CI, 1.01 to 1.57 0.67 53.3 21 Serious adverse event % RR 1.21; 95% CI, 1.00 to 1.45 43.6 Conclusion: Among infants with moderate or severe hypoxic-ischemic encephalopathy receiving therapeutic hypothermia, multiple high doses of erythropoietin did not lower the risk of death or neurodevelopmental impairment & were associated with a greater number of serious adverse events than placebo YW Wu et al. NEJM 2022; 387:148-159