KEYNOTE 671 Trial Summary
Introduction:
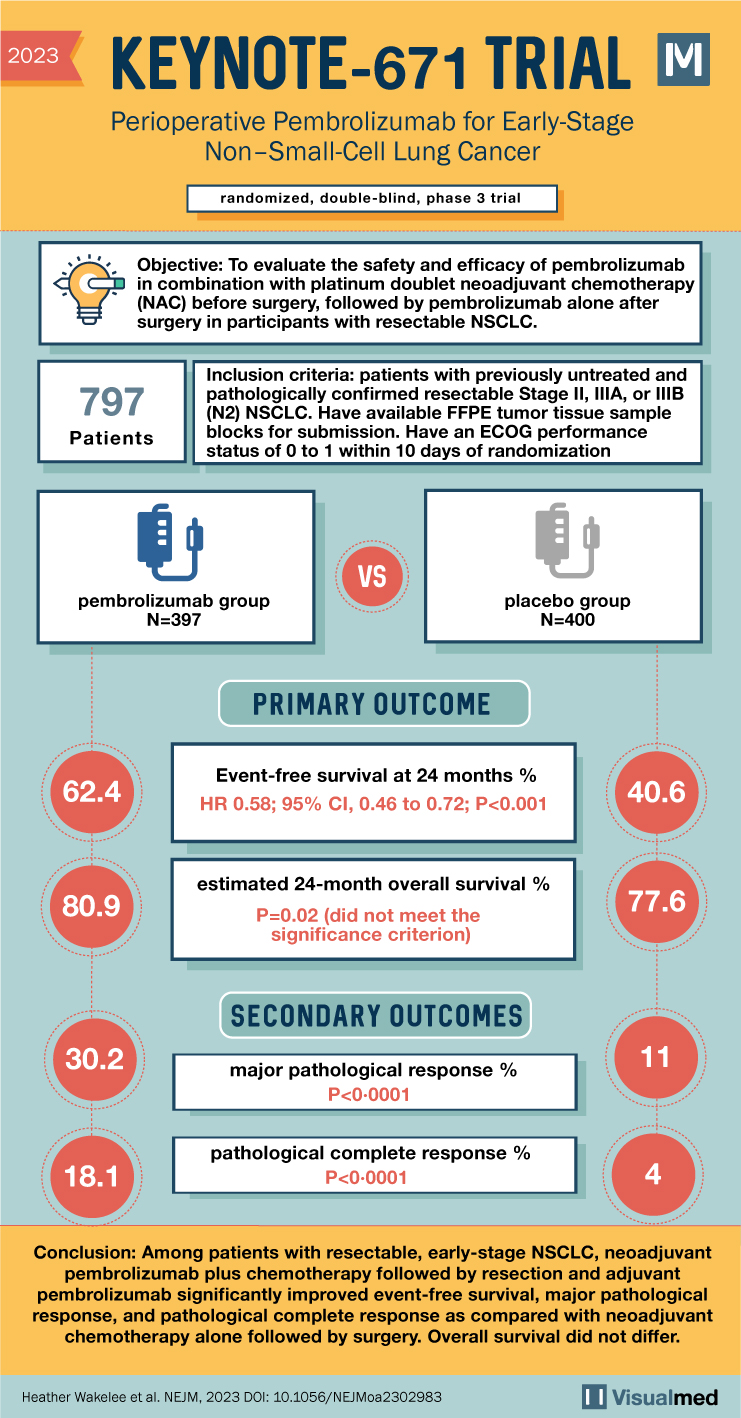
The KEYNOTE 671 trial aimed to investigate the efficacy of perioperative pembrolizumab, an immune checkpoint inhibitor, in patients with resectable early-stage non–small-cell lung cancer (NSCLC). This randomized, double-blind, phase 3 trial assessed the benefits of neoadjuvant and adjuvant pembrolizumab in combination with chemotherapy compared to chemotherapy alone.
Methods:
The trial enrolled participants with resectable stage II, IIIA, or IIIB (N2 stage) NSCLC. Key features of the study design included:
- Treatment Groups: Participants were randomly assigned in a 1:1 ratio to receive either neoadjuvant pembrolizumab (200 mg) or placebo every 3 weeks, along with cisplatin-based chemotherapy for 4 cycles. This was followed by surgery and adjuvant pembrolizumab or placebo every 3 weeks for up to 13 cycles.
- Primary Endpoints: The trial’s primary endpoints were event-free survival (time from randomization to local progression, unresectable tumor, progression/recurrence, or death) and overall survival.
- Secondary Endpoints: Major pathological response, pathological complete response, and safety were among the secondary endpoints assessed.
Inclusion Criteria:
To be eligible for the trial, participants had to meet the following criteria:
- Previously untreated and pathologically confirmed resectable Stage II, IIIA, or IIIB (N2) NSCLC.
- Compliance with contraceptive measures or abstinence for males, and for females, compliance with contraception and agreement not to donate eggs or freeze/store them for reproduction purposes during the treatment period and elimination time of each study intervention.
- Availability of formalin-fixed paraffin-embedded (FFPE) tumor tissue sample blocks or unstained slides for central programmed death-ligand 1 (PD-L1) testing.
- Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 1.
- Adequate organ function.
Exclusion Criteria:
Participants were excluded from the trial if they met any of the following criteria:
- Tumor involvement of the superior sulcus, large cell neuro-endocrine cancer (LCNEC), or sarcomatoid tumor.
- History of pneumonitis/interstitial lung disease requiring steroids or current pneumonitis/interstitial lung disease requiring steroids.
- Active infection requiring systemic therapy.
- History of allogenic tissue/solid organ transplant.
- Severe hypersensitivity to pembrolizumab or any study chemotherapy agents.
- Active autoimmune disease requiring systemic treatment in the past 2 years.
- History of human immunodeficiency virus (HIV) infection, Hepatitis B, Hepatitis C, or active tuberculosis.
- Any condition, therapy, or abnormality that could confound trial results or interfere with participation.
- Psychiatric or substance abuse disorders hindering trial cooperation.
- Prior therapy with anti-PD-1, anti-PD-L1, or anti-PD-L2 agents or agents targeting co-inhibitory T-cell receptors.
- Prior systemic anti-cancer therapy or investigational agents for the current malignancy before randomization.
- Prior radiotherapy within 2 weeks of trial treatment initiation.
- Recent live vaccine administration or participation in another investigational trial or use of investigational devices within 4 weeks prior to trial treatment.
Results:
The KEYNOTE 671 trial analyzed 397 participants in the pembrolizumab group and 400 in the placebo group. Key results from the trial include:
- Event-Free Survival: At 24 months, event-free survival was 62.4% in the pembroliz
umab group compared to 40.6% in the placebo group (hazard ratio 0.58, 95% CI 0.46 to 0.72, P<0.001).
- Overall Survival: The estimated 24-month overall survival was 80.9% in the pembrolizumab group and 77.6% in the placebo group (P=0.02, not meeting significance criterion).
- Pathological Responses: A major pathological response occurred in 30.2% of the pembrolizumab group compared to 11.0% in the placebo group (P<0.0001). Pathological complete response rates were 18.1% and 4.0% respectively (P<0.0001).
- Safety: Grade 3 or higher treatment-related adverse events occurred in 44.9% of the pembrolizumab group and 37.3% of the placebo group.
Conclusions:
Neoadjuvant pembrolizumab plus chemotherapy followed by resection and adjuvant pembrolizumab significantly improved event-free survival, major pathological response, and pathological complete response compared to neoadjuvant chemotherapy alone followed by surgery in resectable, early-stage NSCLC. The overall survival difference did not reach statistical significance in this analysis. The KEYNOTE 671 trial findings provide valuable insights into the potential benefits of perioperative immune checkpoint inhibition in the management of early-stage NSCLC.