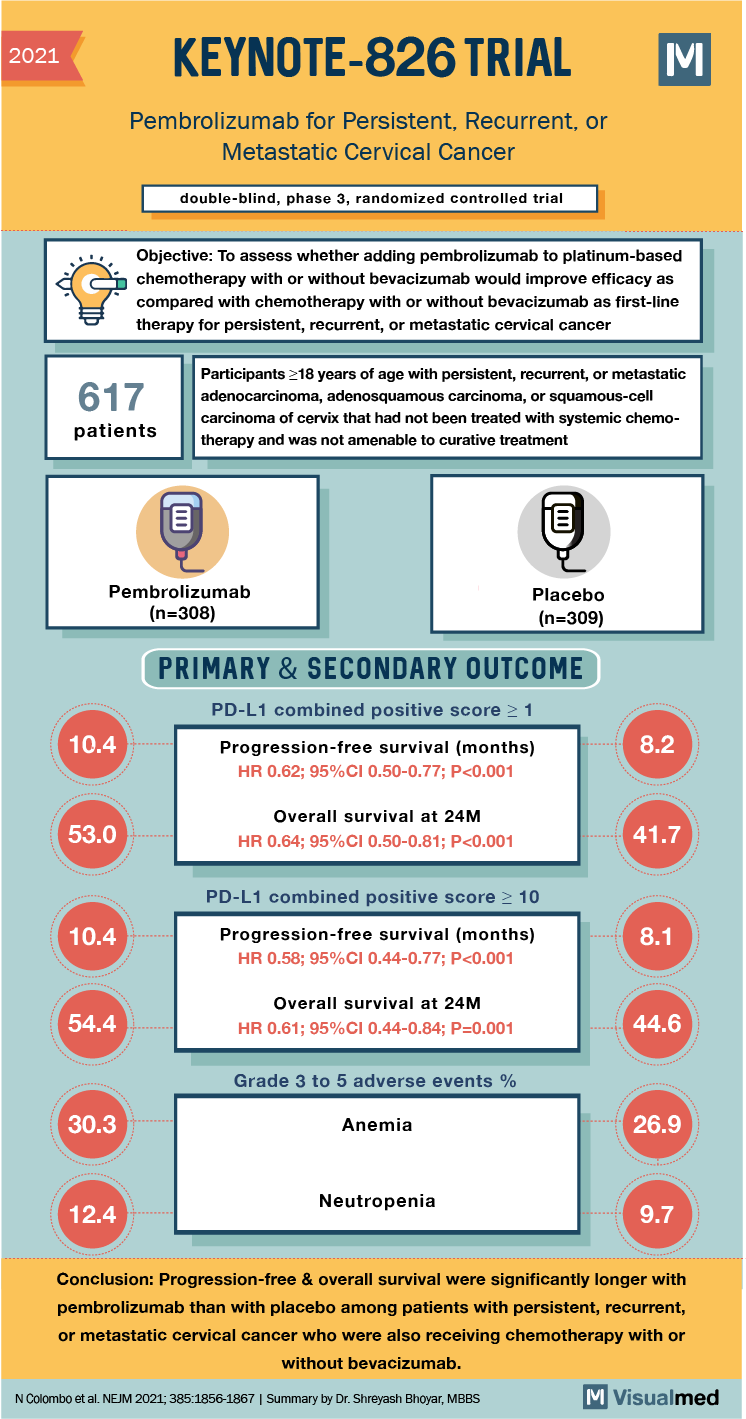
2021 KEYNOTE-826 TRIAL Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer double-blind, phase 3, randomized controlled trial Objective: To assess whether adding pembrolizumab to platinum-based chemotherapy with or without bevacizumab would improve efficacy as compared with chemotherapy with or without bevacizumab as first-line therapy for persistent, recurrent, or metastatic cervical cancer 617 patients Participants 18 years of age with persistent, recurrent, or metastatic adenocarcinoma, adenosquamous carcinoma, or squamous-cell carcinoma of cervix that had not been treated with systemic chemotherapy and was not amenable to curative treatment Pembrolizumab (n=308) Placebo (n=309) PRIMARY & SECONDARY OUTCOME PD-L1 combined positive score > 1 10.4 8.2 Progression-free survival (months) HR 0.62; 95%CI 0.50-0.77; P<0.001 53.0 Overall survival at 24M HR 0.64; 95%CI 0.50-0.81; P<0.001 41.7 PD-L1 combined positive score > 10 10.4 Progression-free survival (months) HR 0.58; 95%CI 0.44-0.77; P<0.001 8.1 54.4 Overall survival at 24M HR 0.61; 95%CI 0.44-0.84; P=0.001 44.6 Grade 3 to 5 adverse events % 30.3 Anemia 26.9 Neutropenia 12.4 9.7 Conclusion: Progression-free & overall survival were significantly longer with pembrolizumab than with placebo among patients with persistent, recurrent, or metastatic cervical cancer who were also receiving chemotherapy with or without bevacizumab. N Colombo et al. NEJM 2021;385:1856-1867