- Year: 2024
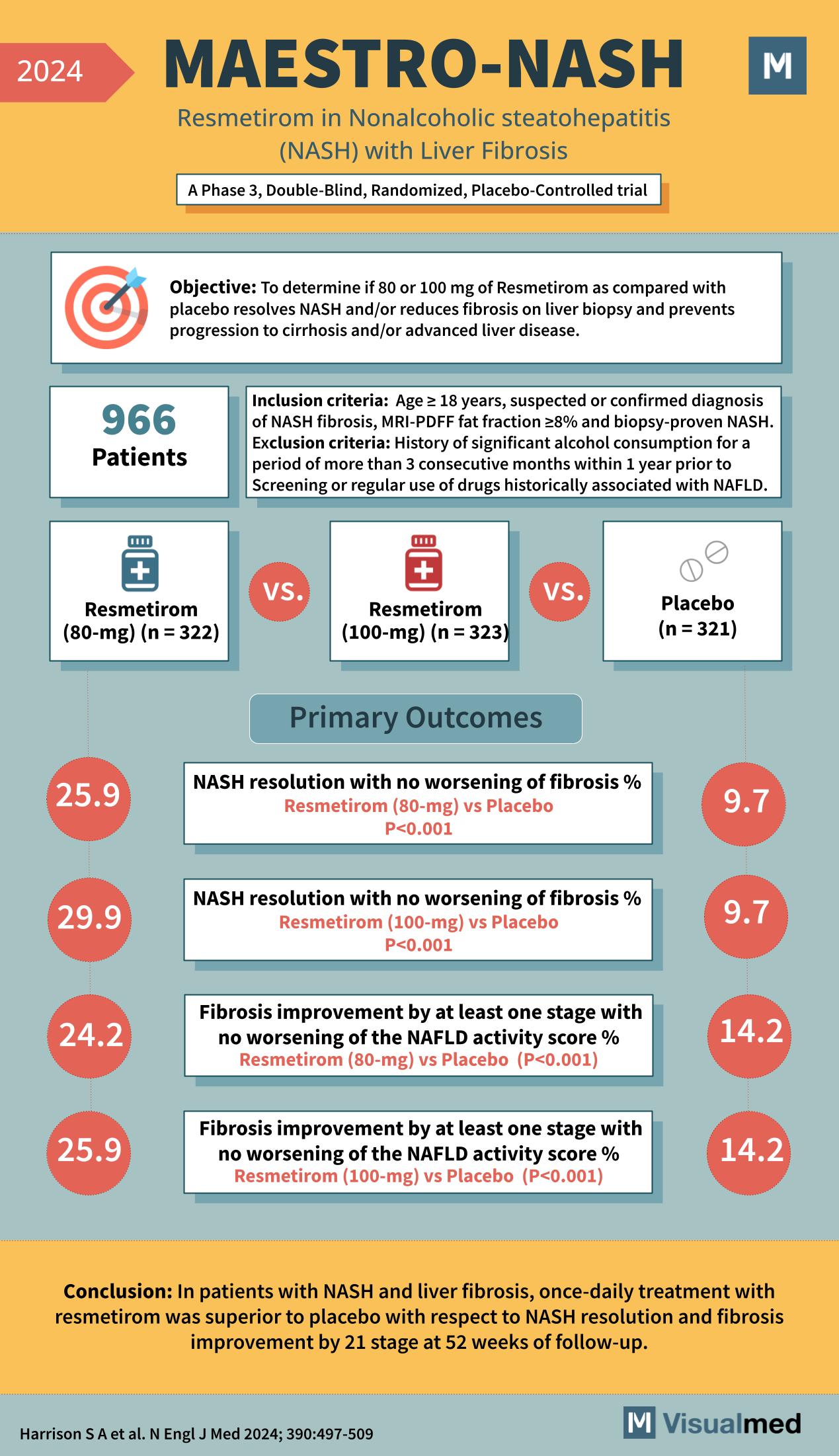
- Title: MAESTRO-NASH
- Subtitle: Resmetirom in Nonalcoholic steatohepatitis (NASH) with Liver Fibrosis
- Trial Type: A Phase 3, Double-Blind, Randomized, Placebo-Controlled trial
Objective: To determine if 80 or 100 mg of Resmetirom compared with placebo resolves NASH and/or reduces fibrosis on liver biopsy and prevents progression to cirrhosis and/or advanced liver disease.
Inclusion Criteria:
- Age ≥ 18 years
- Suspected or confirmed diagnosis of NASH fibrosis
- MRI-PDFF fat fraction ≥8% and biopsy-proven NASH
Exclusion Criteria:
- History of significant alcohol consumption for a period of more than 3 consecutive months within 1 year prior to screening
- Regular use of drugs historically associated with NAFLD
Participants: 966 Patients
Treatment Groups:
- Resmetirom (80-mg) – 322 patients
- Resmetirom (100-mg) – 323 patients
- Placebo – 321 patients
Primary Outcomes:
- NASH resolution with no worsening of fibrosis %: Resmetirom (80-mg) vs Placebo – 25.9 vs 9.7 (P<0.001)
- NASH resolution with no worsening of fibrosis %: Resmetirom (100-mg) vs Placebo – 29.9 vs 9.7 (P<0.001)
- Fibrosis improvement by at least one stage with no worsening of the NAFLD activity score %: Resmetirom (80-mg) vs Placebo – 24.2 vs 14.2 (P<0.001)
- Fibrosis improvement by at least one stage with no worsening of the NAFLD activity score %: Resmetirom (100-mg) vs Placebo – 25.9 vs 14.2 (P<0.001)
Conclusion: In patients with NASH and liver fibrosis, once-daily treatment with resmetirom was superior to placebo with respect to NASH resolution and fibrosis improvement by 21 stage at 52 weeks of follow-up.
Reference: Harrison S A et al. N Engl J Med 2024; 390:497-509