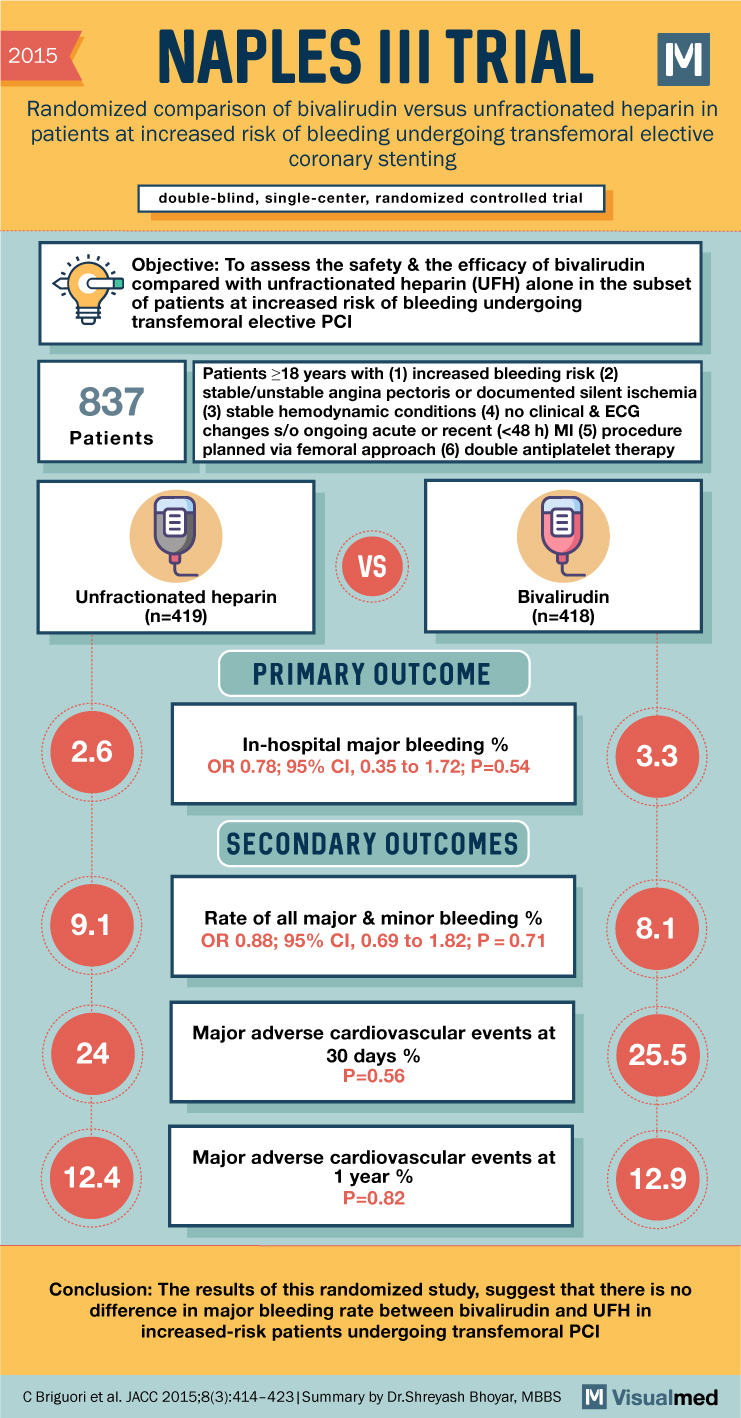
2015 NAPLES III TRIAL Randomized comparison of bivalirudin versus unfractionated heparin in patients at increased risk of bleeding undergoing transfemoral elective coronary stenting double-blind, single-center, randomized controlled trial Objective: To assess the safety & the efficacy of bivalirudin compared with unfractionated heparin (UFH) alone in the subset of patients at increased risk of bleeding undergoing transfemoral elective PCI 837 Patients Patients ≥18 years with (1) increased bleeding risk (2) stable/unstable angina pectoris or documented silent ischemia (3) stable hemodynamic conditions (4) no clinical & ECG changes s/o ongoing acute or recent (<48 h) MI (5) procedure planned via femoral approach (6) double antiplatelet therapy 目 VS Unfractionated heparin (n=419) 目 PRIMARY OUTCOME Bivalirudin (n=418) 2.6 In-hospital major bleeding % OR 0.78; 95% CI, 0.35 to 1.72; P=0.54 3.3 SECONDARY OUTCOMES 9.1 Rate of all major & minor bleeding % OR 0.88; 95% CI, 0.69 to 1.82; P = 0.71 8.1 Major adverse cardiovascular events at 24 30 days % P=0.56 25.5 12.4 Major adverse cardiovascular events at 1 year % P=0.82 12.9 Conclusion: The results of this randomized study, suggest that there is no difference in major bleeding rate between bivalirudin and UFH in increased-risk patients undergoing transfemoral PCI C Briguori et al. JACC 2015;8(3):414-423