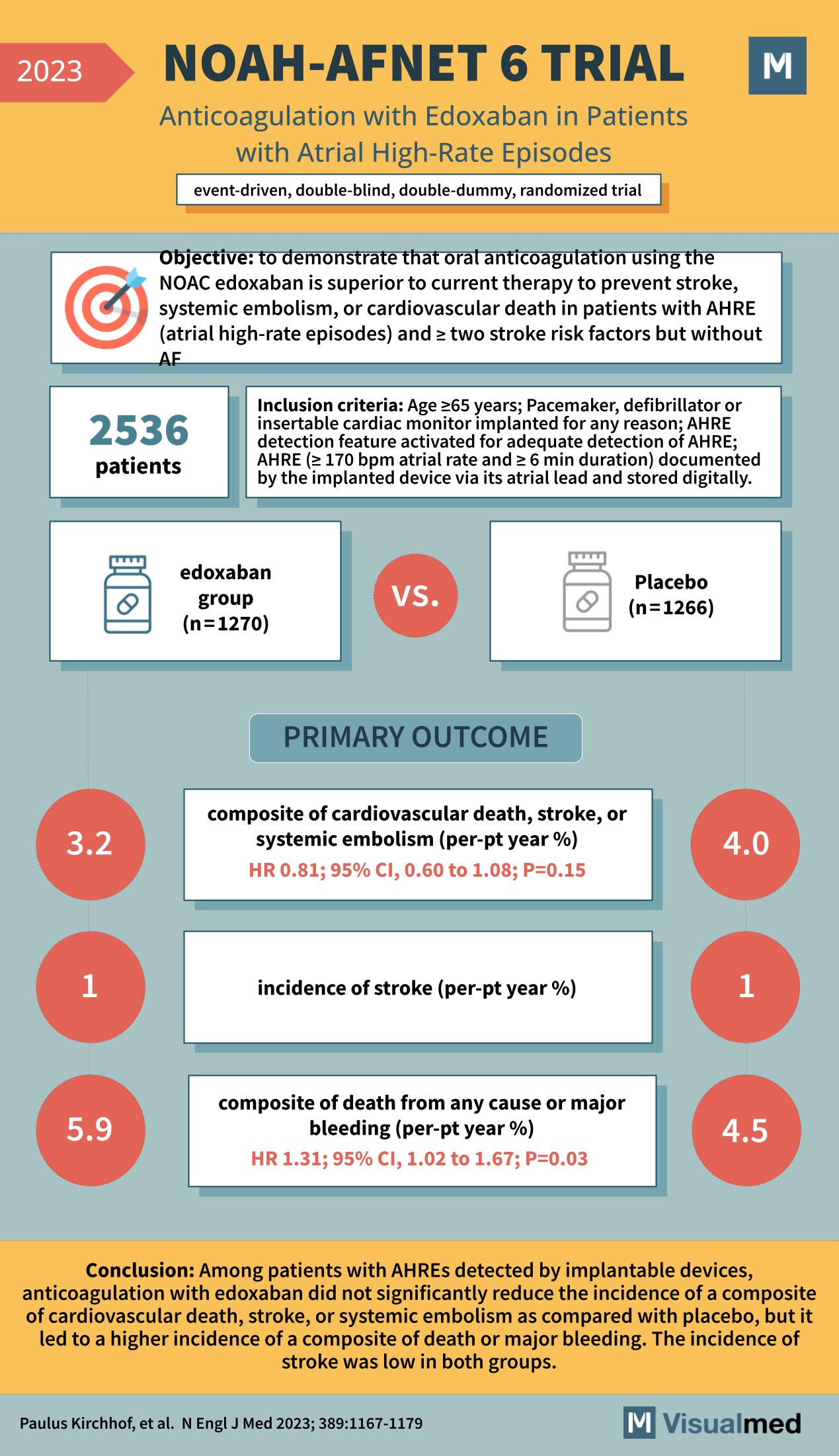
The NOAH-AFNET 6 trial, as outlined in a groundbreaking study from the New England Journal of Medicine in 2023, represents a significant advancement in our understanding of atrial fibrillation management. This study scrutinized the efficacy of edoxaban, a novel oral anticoagulant (NOAC), against placebo in preventing severe cardiovascular outcomes in patients with atrial high-rate episodes (AHRE) but without atrial fibrillation (AF).
With 2536 patients enrolled, the NOAH-AFNET 6 trial was structured as an event-driven, double-blind, double-dummy, randomized trial. The study’s objective was to demonstrate that edoxaban is superior to current therapy to prevent stroke, systemic embolism, or cardiovascular death in patients with AHRE and two or more stroke risk factors but without AF. Participants were aged 65 years or older and had a pacemaker, defibrillator, or insertable cardiac monitor implanted for any reason, documenting AHRE with a specific criteria of ≥170 bpm atrial rate and ≥6 min duration.
The primary outcome of interest was a composite of cardiovascular death, stroke, or systemic embolism. The edoxaban group showed a 3.2% incidence of this composite outcome, compared to 4.0% in the placebo group, yielding a hazard ratio (HR) of 0.81 with a 95% confidence interval (CI) of 0.60 to 1.08; however, this did not reach statistical significance with a p-value of 0.15.
Furthermore, the trial reported the incidence of stroke, which was 1% in both the edoxaban group and the placebo group, indicating no significant difference between the two treatments in preventing strokes. The secondary outcome, which was a composite of death from any cause or major bleeding, showed a higher incidence in the edoxaban group at 5.9% versus 4.5% in the placebo group. This resulted in an HR of 1.31, suggesting that while edoxaban may not significantly reduce the incidence of the composite of cardiovascular death, stroke, or systemic embolism, it could lead to an increased risk of major bleeding, with statistical significance (p=0.03).
The conclusion of the NOAH-AFNET 6 trial was particularly noteworthy. Among patients with AHREs detected by implantable devices, anticoagulation with edoxaban did not significantly reduce the incidence of the primary composite outcome when compared with placebo. However, there was a higher incidence of a composite of death or major bleeding, although the overall incidence of stroke remained low in both groups.
The NOAH-AFNET 6 trial presents a nuanced perspective on the use of NOACs like edoxaban in a subset of patients who are traditionally challenging to treat due to the absence of manifest AF. The findings prompt a careful consideration of the risk-benefit ratio in using anticoagulation therapy in patients with AHREs, especially given the increased risk of major bleeding.
This trial has undoubtedly added to the collective medical knowledge, influencing guidelines and clinical practices regarding stroke prevention in patients with cardiac rhythm abnormalities. The NOAH-AFNET 6 trial’s results emphasize the importance of individualized patient care and the need for continued research to optimize treatment strategies for patients with AHREs. The insights from this trial are critical for cardiologists, primary care physicians, and other healthcare providers involved in the management of cardiovascular disease, especially in an era where precision medicine is becoming increasingly paramount.