“Innovations in NSCLC Treatment: The AEGEAN Trial Breakthrough” In recent years, the pursuit of more effective treatments for non-small-cell lung cancer (NSCLC) has been relentless. The AEGEAN trial of 2023, as reported in the esteemed New England Journal of Medicine (NEJM), heralds a significant step forward in this quest. This phase III, randomized, double-blind, placebo-controlled trial dives deep into the efficacy of perioperative durvalumab combined with chemotherapy for resectable NSCLC. Let’s unpack the key findings of this groundbreaking study and what it means for future NSCLC treatment.
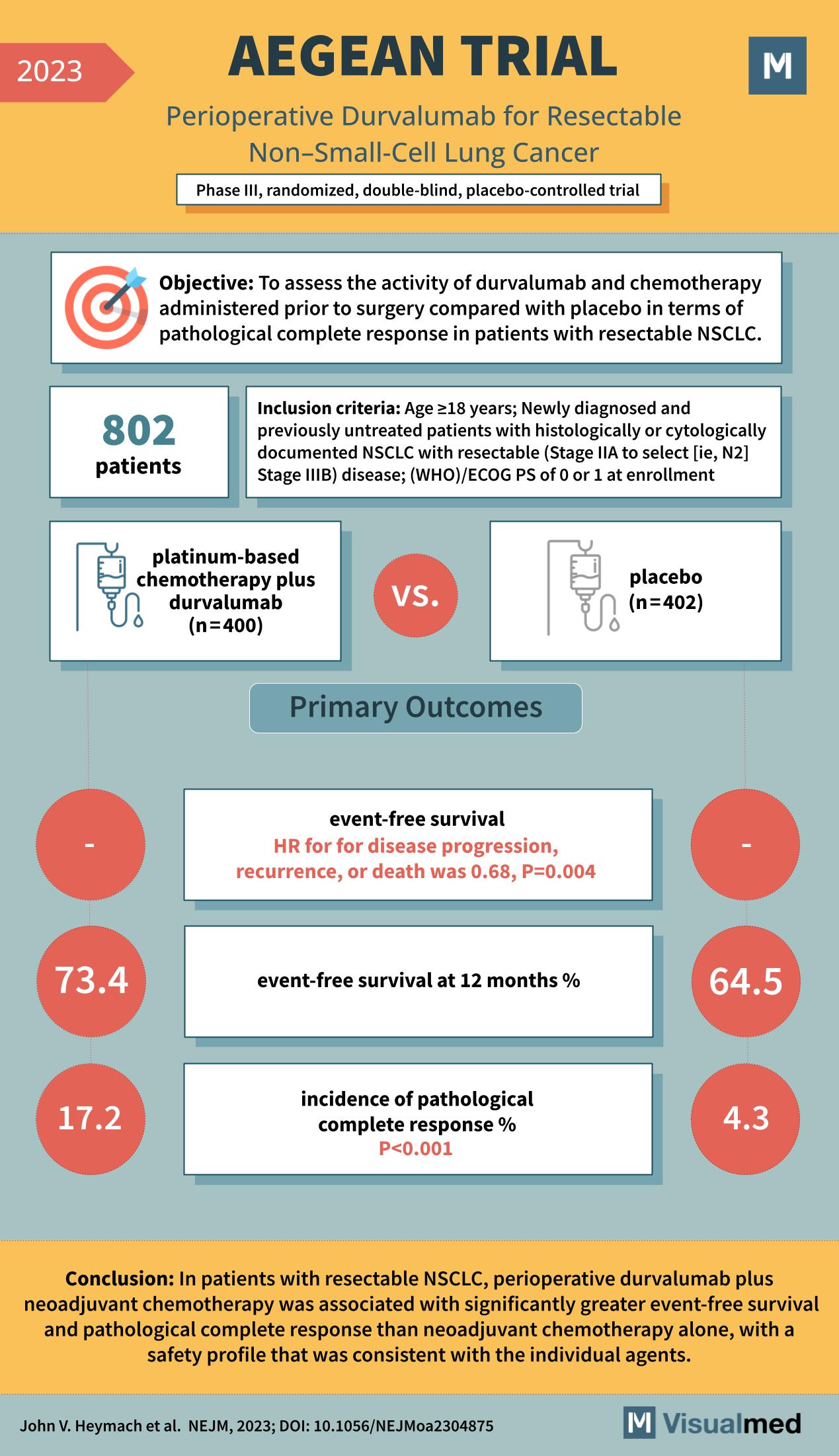
What is the AEGEAN Trial? The AEGEAN trial was set out with a primary objective to gauge the activity of durvalumab (an immunotherapy agent) and chemotherapy administered before surgery. It aimed to measure its effectiveness compared with a placebo in achieving a pathological complete response in patients with resectable NSCLC. This is crucial because a higher pathological complete response rate often translates to better long-term outcomes for cancer patients.
Study Parameters A robust 802 patients, aged 18 years and above, participated in the study. All of these patients were newly diagnosed and had not previously undergone treatment. They were diagnosed either histologically or cytologically with NSCLC that was deemed resectable, ranging from Stage IIA to select Stage IIIB. Further refining the participant criteria, the study included only those with a World Health Organization/Eastern Cooperative Oncology Group performance status (WHO/ECOG PS) of 0 or 1 at enrollment. Treatment Regimes Patients were divided into two main groups. The first, comprising 400 patients, received a combination of platinum-based chemotherapy plus durvalumab. The second group, with 402 patients, was administered a placebo.
Outcomes & Results The primary outcomes focused on event-free survival and the incidence of pathological complete response. Here’s what the trial discovered:
- Event-Free Survival: At the 12-month mark, the group that received durvalumab plus chemotherapy showcased a 73.4% event-free survival rate. In contrast, the placebo group’s rate stood at 64.5%. Furthermore, the hazard ratio for disease progression, recurrence, or death was an encouraging 0.68 with a statistically significant p-value of 0.004.
- Pathological Complete Response: The durvalumab group had an impressive 17.2% incidence of achieving a pathological complete response. This was notably higher than the 4.3% observed in the placebo group, with a highly significant p-value of less than 0.001.
Concluding Thoughts
The AEGEAN trial’s conclusion is compelling. For patients with resectable NSCLC, perioperative durvalumab combined with neoadjuvant chemotherapy was associated with notably better event-free survival and pathological complete response rates than chemotherapy alone. Moreover, the safety profile remained consistent with the known profiles of the individual agents used. This study’s results promise a brighter horizon for NSCLC patients, as it emphasizes the potential benefits of combining immunotherapy agents like durvalumab with standard chemotherapy regimens. As we continue to delve into the vast realm of cancer research, studies like AEGEAN illuminate the path forward, offering hope and tangible results for patients worldwide.