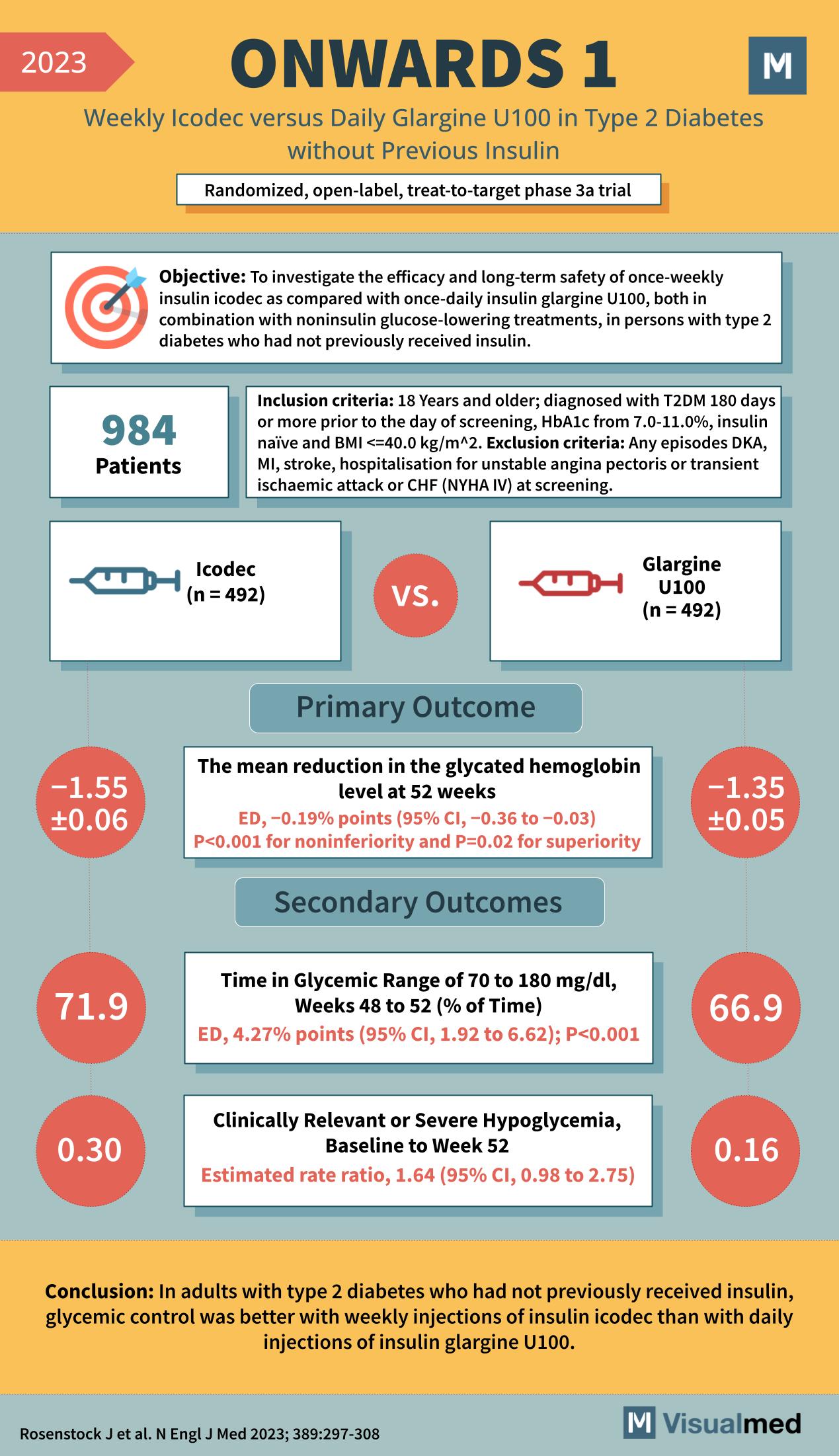
Year: 2023 Title: ONWARDS 1 Subtitle: Weekly Icodec versus Daily Glargine U100 in Type 2 Diabetes without Previous Insulin Type of Trial: Randomized, open-label, treat-to-target phase 3a trial
Objective: To investigate the efficacy and long-term safety of once-weekly insulin icodec compared with once-daily insulin glargine U100, both in combination with noninsulin glucose-lowering treatments, in persons with type 2 diabetes who had not previously received insulin.
Patients: 984
Inclusion Criteria:
- 18 Years and older
- Diagnosed with T2DM 180 days or more prior to the day of screening
- HbA1c from 7.0-11.0%
- Insulin naïve and BMI ≤40.0 kg/m^2
Exclusion Criteria:
- Any episodes DKA, MI, stroke, hospitalization for unstable angina pectoris or transient ischemic attack or CHF (NYHA IV) at screening.
Groups:
- Icodec (n = 492)
- Glargine U100 (n = 492)
Primary Outcome:
- The mean reduction in the glycated hemoglobin level at 52 weeks
- Icodec: -1.55 ± 0.06
- Glargine U100: -1.35 ± 0.05
- Estimated Difference (ED), -0.19 percentage points (95% CI, -0.36 to -0.03); P<0.001 for noninferiority and P=0.02 for superiority
Secondary Outcomes:
- Time in Glycemic Range of 70 to 180 mg/dl, Weeks 48 to 52 (% of Time)
- Icodec: 71.9%
- Glargine U100: 66.9%
- ED, 4.27 percentage points (95% CI, 1.92 to 6.62); P<0.001
- Clinically Relevant or Severe Hypoglycemia, Baseline to Week 52
- Icodec: 0.30%
- Glargine U100: 0.16%
- Estimated rate ratio, 1.64 (95% CI, 0.98 to 2.75)
Conclusion: In adults with type 2 diabetes who had not previously received insulin, glycemic control was better with weekly injections of insulin icodec than with daily injections of insulin glargine U100.
Reference: Rosenstock J et al. N Engl J Med 2023; 389:297-308