The PACER Trial: Revisiting Platelet Transfusions for CVC Placement
The 2023 PACER Trial investigated a critical aspect of clinical care involving patients with thrombocytopenia undergoing central venous catheter (CVC) placement. This parallel, single-blinded, randomized controlled trial aimed to establish whether prophylactic platelet transfusion could be safely omitted before CVC placement.
Objective of the PACER Trial
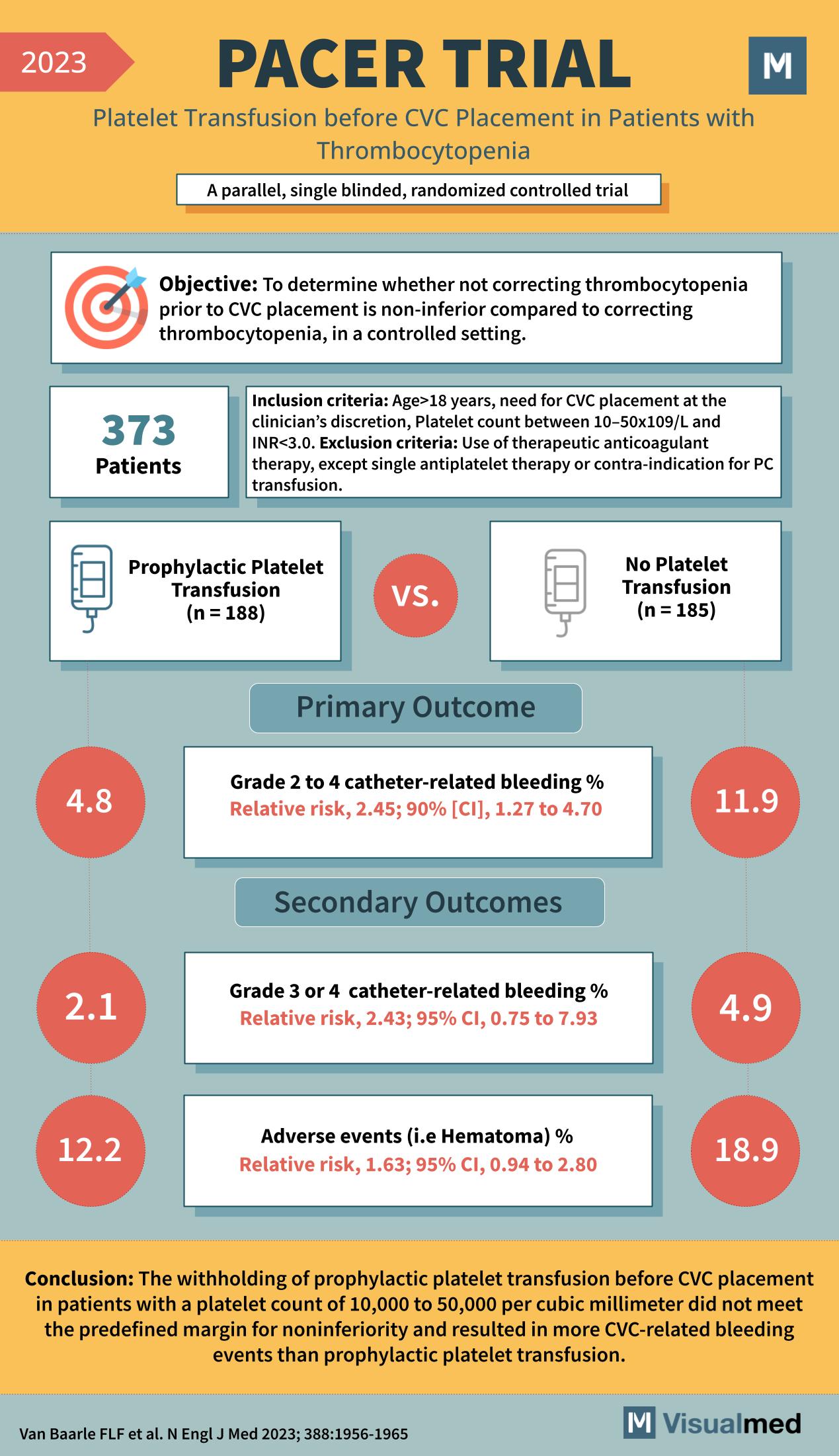
The study’s goal was to determine the non-inferiority of not correcting thrombocytopenia before CVC placement compared to the standard practice of correcting it in a controlled setting.
Trial Design and Participant Criteria
In total, 373 patients were enrolled. These individuals were all 18 years or older, had a platelet count of 10–50×10^9/L, and were indicated for CVC placement, without being on therapeutic anticoagulant therapy or contraindications for platelet transfusion.
Interventions and Comparison
Participants were divided into two groups: one received prophylactic platelet transfusions (188 patients) while the other did not receive platelet transfusions (185 patients) prior to CVC insertion.
Outcomes of the PACER Trial
Primary Outcome
- There was a higher percentage of grade 2 to 4 catheter-related bleeding in the group that did not receive platelet transfusions (11.9%) compared to the group that did (4.8%), with a relative risk of 2.45.
Secondary Outcomes
- For more severe grade 3 or 4 catheter-related bleeding, the no-transfusion group had a relative risk of 2.43.
- The group that did not receive transfusions also had a higher percentage of adverse events like hematomas (18.9%) compared to the transfusion group (12.2%), with a relative risk of 1.63.
Conclusion and Implications for Clinical Practice
The PACER Trial concluded that withholding prophylactic platelet transfusion before CVC placement in patients with a platelet count of 10,000 to 50,000 per cubic millimeter did not meet the predefined margin for noninferiority. It resulted in more CVC-related bleeding events than the group receiving prophylactic platelet transfusions. These findings have significant implications for patient safety and clinical protocols surrounding invasive procedures in thrombocytopenic patients.