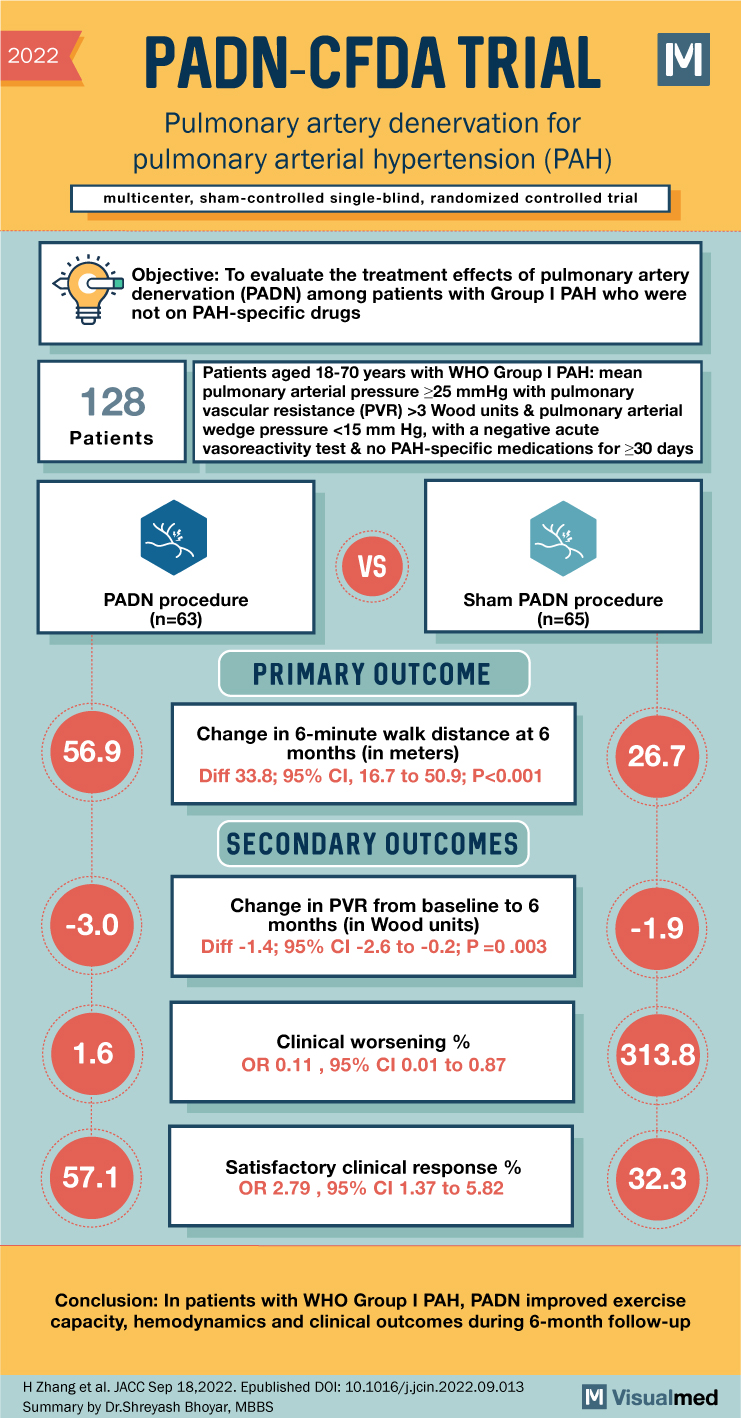
2022 PADN-CFDA TRIAL Pulmonary artery denervation for pulmonary arterial hypertension (PAH) multicenter, sham-controlled single-blind, randomized controlled trial Objective: To evaluate the treatment effects of pulmonary artery denervation (PADN) among patients with Group I PAH who were not on PAH-specific drugs 128 Patients Patients aged 18-70 years with WHO Group I PAH: mean pulmonary arterial pressure >25 mmHg with pulmonary vascular resistance (PVR) >3 Wood units & pulmonary arterial wedge pressure <15 mm Hg, with a negative acute vasoreactivity test & no PAH-specific medications for >30 days VS PADN procedure (n=63) Sham PADN procedure (n=65) 56.9 -3.0 PRIMARY OUTCOME Change in 6-minute walk distance at 6 months (in meters) Diff 33.8; 95% CI, 16.7 to 50.9; P<0.001 SECONDARY OUTCOMES Change in PVR from baseline to 6 months (in Wood units) Diff -1.4; 95% CI -2.6 to -0.2; P=0.003 26.7 -1.9 1.6 Clinical worsening % 313.8 OR 0.11, 95% CI 0.01 to 0.87 57.1 Satisfactory clinical response % OR 2.79, 95% CI 1.37 to 5.82 32.3 Conclusion: In patients with WHO Group I PAH, PADN improved exercise capacity, hemodynamics and clinical outcomes during 6-month follow-up H Zhang et al. JACC Sep 18,2022. Epublished DOI: 10.1016/j.jcin.2022.09.013