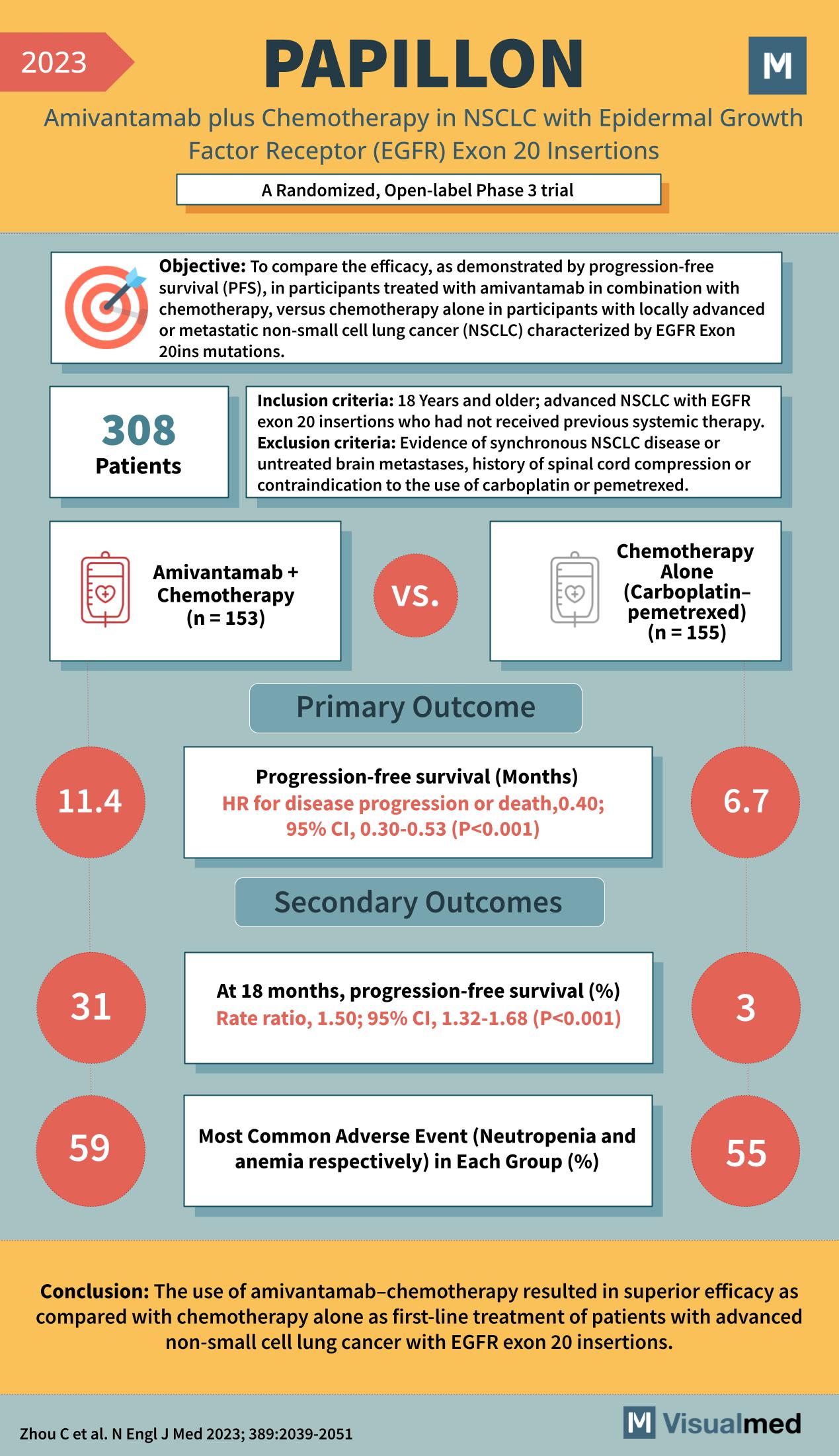
Year: 2023 Title: PAPILLON Subtitle: Amivantamab plus Chemotherapy in NSCLC with Epidermal Growth Factor Receptor (EGFR) Exon 20 Insertions Type of Trial: A Randomized, Open-label Phase 3 trial
Objective: To compare the efficacy, as demonstrated by progression-free survival (PFS), in participants treated with amivantamab in combination with chemotherapy, versus chemotherapy alone in participants with locally advanced or metastatic non-small cell lung cancer (NSCLC) characterized by EGFR Exon 20ins mutations.
Patients: 308
Inclusion Criteria:
- 18 Years and older
- Advanced NSCLC with EGFR exon 20 insertions
- Not received previous systemic therapy
Exclusion Criteria:
- Evidence of synchronous NSCLC disease or untreated brain metastases
- History of spinal cord compression
- Contraindication to the use of carboplatin or pemetrexed
Groups:
- Amivantamab + Chemotherapy (n = 153)
- Chemotherapy Alone (Carboplatin-pemetrexed) (n = 155)
Primary Outcome:
- Progression-free survival (Months)
- Amivantamab + Chemotherapy: 11.4 months
- Chemotherapy Alone: 6.7 months
- HR for disease progression or death, 0.40; 95% CI, 0.30-0.53 (P<0.001)
Secondary Outcomes:
- At 18 months, progression-free survival (%)
- Amivantamab + Chemotherapy: 31%
- Chemotherapy Alone: 3%
- Rate ratio, 1.50; 95% CI, 1.32-1.68 (P<0.001)
- Most Common Adverse Event (Neutropenia and anemia respectively) in Each Group (%)
- Amivantamab + Chemotherapy: 59%
- Chemotherapy Alone: 55%
Conclusion: The use of amivantamab-chemotherapy resulted in superior efficacy compared with chemotherapy alone as first-line treatment of patients with advanced non-small cell lung cancer with EGFR exon 20 insertions.
Reference: Zhou C et al. N Engl J Med 2023; 389:2039-2051