High levels of triglycerides, a type of fat found in the blood, have been connected to an increased risk of cardiovascular diseases. The question of whether decreasing these levels can reduce the incidence of cardiovascular events has been under investigation. Pemafibrate, a selective peroxisome proliferator–activated receptor α modulator, has been shown to lower triglyceride levels and improve other lipid levels.
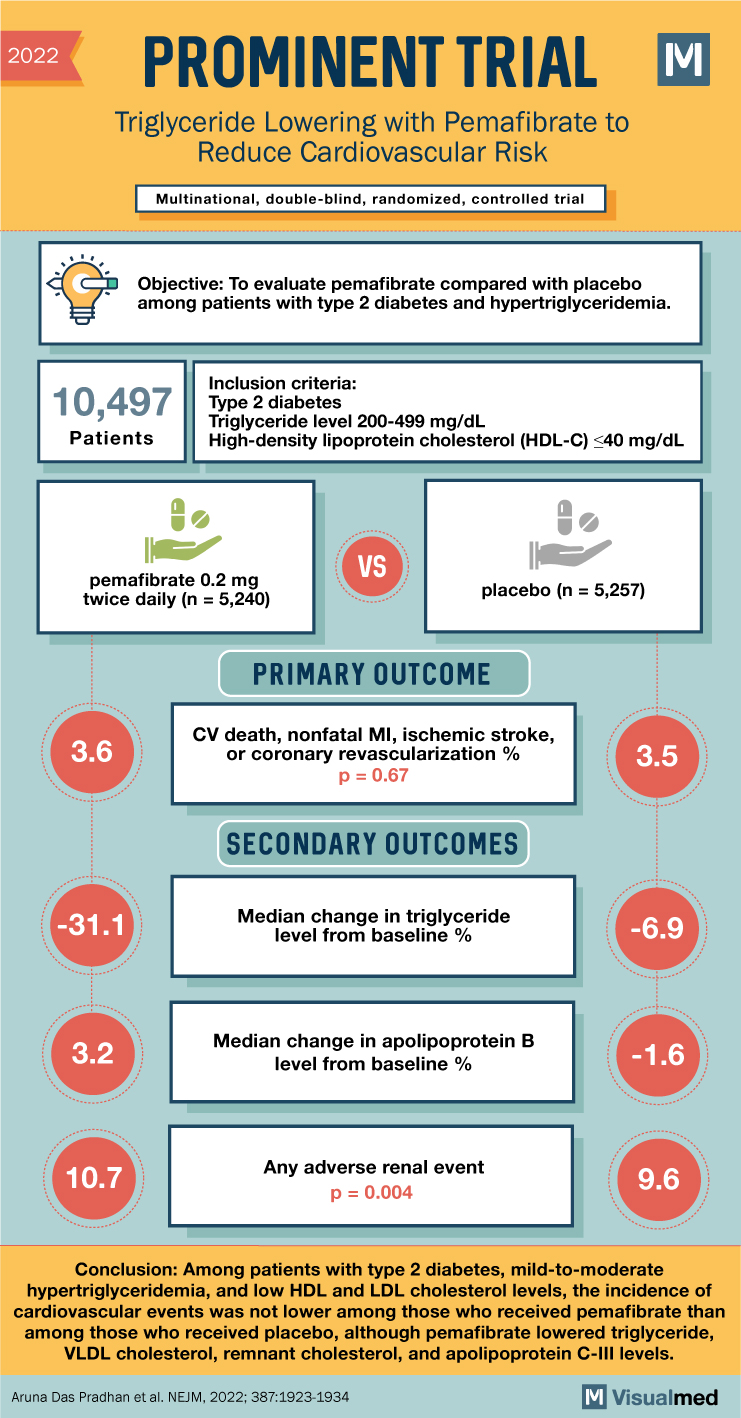
In an international, double-blind, randomized, controlled trial involving 10,497 patients with type 2 diabetes, mild-to-moderate hypertriglyceridemia (triglyceride levels of 200 to 499 mg per deciliter), and low high-density lipoprotein (HDL) cholesterol levels (40 mg per deciliter or lower), the effects of pemafibrate were examined. Participants were either assigned to receive pemafibrate or a matching placebo. These patients were already receiving guideline-directed lipid-lowering therapy, or couldn’t undergo statin therapy due to adverse effects, and had low-density lipoprotein (LDL) cholesterol levels of 100 mg per deciliter or lower. The primary efficacy end point was a composite of nonfatal myocardial infarction, ischemic stroke, coronary revascularization, or death from cardiovascular causes.
The study followed patients for a median period of 3.4 years. After 4 months, pemafibrate had resulted in a decrease of 26.2% for triglycerides, 25.8% for very-low-density lipoprotein (VLDL) cholesterol, 25.6% for remnant cholesterol, 27.6% for apolipoprotein C-III, and an increase of 4.8% for apolipoprotein B. However, the primary end-point event occurred in 572 patients in the pemafibrate group and in 560 of those in the placebo group (hazard ratio, 1.03; 95% confidence interval, 0.91 to 1.15), demonstrating no significant reduction in cardiovascular events despite the improved lipid levels.
The overall incidence of serious adverse events was similar between the two groups. However, pemafibrate was associated with a higher incidence of adverse renal events and venous thromboembolism, and a lower incidence of nonalcoholic fatty liver disease.
In conclusion, despite reducing triglyceride, VLDL cholesterol, remnant cholesterol, and apolipoprotein C-III levels, pemafibrate did not lower the incidence of cardiovascular events among patients with type 2 diabetes, mild-to-moderate hypertriglyceridemia, and low HDL and LDL cholesterol levels. This suggests that reducing these lipid levels alone may not be sufficient to lower cardiovascular risk, and additional strategies may need to be explored.