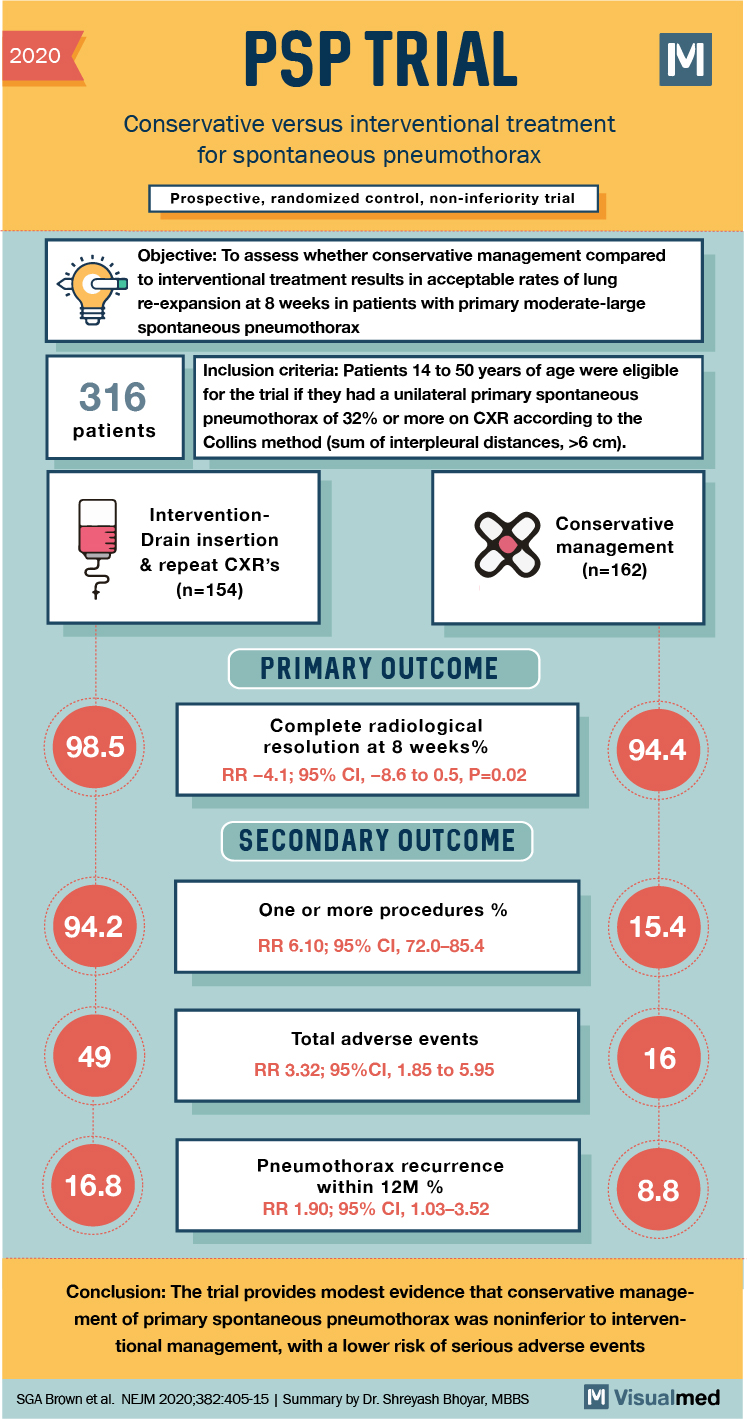
2020 PSP TRIAL M Conservative versus interventional treatment for spontaneous pneumothorax Prospective, randomized control, non-inferiority trial Objective: To assess whether conservative management compared to interventional treatment results in acceptable rates of lung re-expansion at 8 weeks in patients with primary moderate-large spontaneous pneumothorax 316 patients Inclusion criteria: Patients 14 to 50 years of age were eligible for the trial if they had a unilateral primary spontaneous pneumothorax of 32% or more on CXR according to the Collins method (sum of interpleural distances, >6 cm). InterventionDrain insertion & repeat CXR’s (n=154) Conservative management (n=162) PRIMARY OUTCOME 98.5 Complete radiological resolution at 8 weeks% RR -4.1; 95% CI, -8.6 to 0.5, P=0.02 94.4 SECONDARY OUTCOME 94.2 One or more procedures % RR 6.10; 95% CI, 72.0–85.4 15.4 Total adverse events RR 3.32; 95%CI, 1.85 to 5.95 16.8 Pneumothorax recurrence within 12M % RR 1.90; 95% CI, 1.03-3.52 8.8 Conclusion: The trial provides modest evidence that conservative management of primary spontaneous pneumothorax was noninferior to interventional management, with a lower risk of serious adverse events SGA Brown et al. NEJM 2020;382:405-15