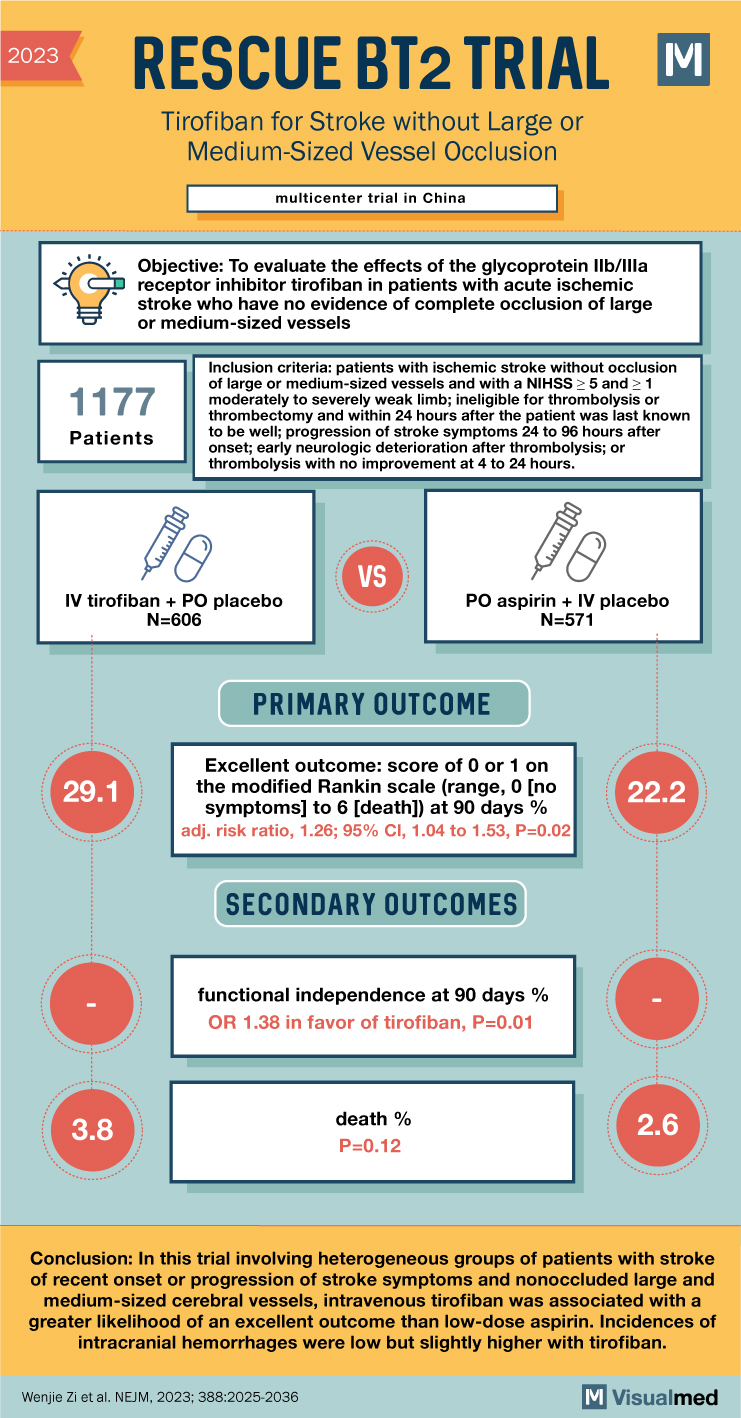
Summary of the RESCUE BT2 Trial: Tirofiban in Acute Ischemic Stroke
Background:
- Limited research on the effects of tirofiban, a glycoprotein IIb/IIIa receptor inhibitor, in acute ischemic stroke patients without complete occlusion of large or medium-sized vessels.
Methods:
- Multicenter trial conducted in China.
- Enrolled patients with ischemic stroke without vessel occlusion, National Institutes of Health Stroke Scale (NIHSS) score of 5 or more, and at least one moderately to severely weak limb.
- Eligible patients had different clinical presentations: ineligible for thrombolysis or thrombectomy within 24 hours of last known well; stroke symptom progression 24 to 96 hours after onset; early neurological deterioration after thrombolysis; or no improvement after thrombolysis at 4 to 24 hours.
- Patients randomized to receive intravenous tirofiban (plus oral placebo) or oral aspirin (100 mg/day plus intravenous placebo) for 2 days, followed by oral aspirin until day 90.
- Primary efficacy endpoint: excellent outcome (modified Rankin scale score of 0 or 1) at 90 days.
- Secondary endpoints: functional independence at 90 days, quality-of-life score.
- Primary safety endpoints: death and symptomatic intracranial hemorrhage.
Inclusion criteria:
- Aged 18 years or older.
- Various presentations of acute ischemic stroke (AIS) within specific timeframes.
- NIHSS score of 5 or more, with at least one limb affected.
- No visible occlusion of large or medium intracranial vessels on imaging.
- Written informed consent obtained.
Results:
- 606 patients in the tirofiban group and 571 patients in the aspirin group.
- Majority had small atherosclerotic infarctions.
- Excellent outcome (mRS score 0 or 1) at 90 days: 29.1% in the tirofiban group vs. 22.2% in the aspirin group (adjusted risk ratio 1.26; 95% CI 1.04-1.53; p=0.02).
- Secondary endpoints did not consistently align with the primary analysis.
- Similar mortality rates in both groups.
- Symptomatic intracranial hemorrhage incidence: 1.0% in tirofiban group vs. 0% in aspirin group.
Conclusions:
- In this trial involving patients with recent onset or progressing stroke symptoms and nonoccluded large or medium-sized cerebral vessels, intravenous tirofiban showed a greater likelihood of excellent outcomes compared to low-dose aspirin.
- Incidence of intracranial hemorrhage was low but slightly higher with tirofiban.
Key Takeaways:
- Tirofiban, a glycoprotein IIb/IIIa receptor inhibitor, showed promise in improving outcomes for acute ischemic stroke patients without complete vessel occlusion.
- Tirofiban was associated with a higher likelihood of excellent outcomes compared to aspirin.
- Incidence of symptomatic intracranial hemorrhage was low but slightly higher with tirofiban.
- Further research is needed to validate these findings and determine the optimal treatment approach for this patient population.