The RUBY Trial: A New Chapter in Endometrial Cancer Treatment
Recent results from the RUBY Trial have introduced a significant advancement in the treatment of primary advanced or recurrent endometrial cancer. The phase 3, randomized, double-blind trial meticulously evaluated the efficacy and safety of Jemperli (dostarlimab) in combination with standard-of-care chemotherapy.
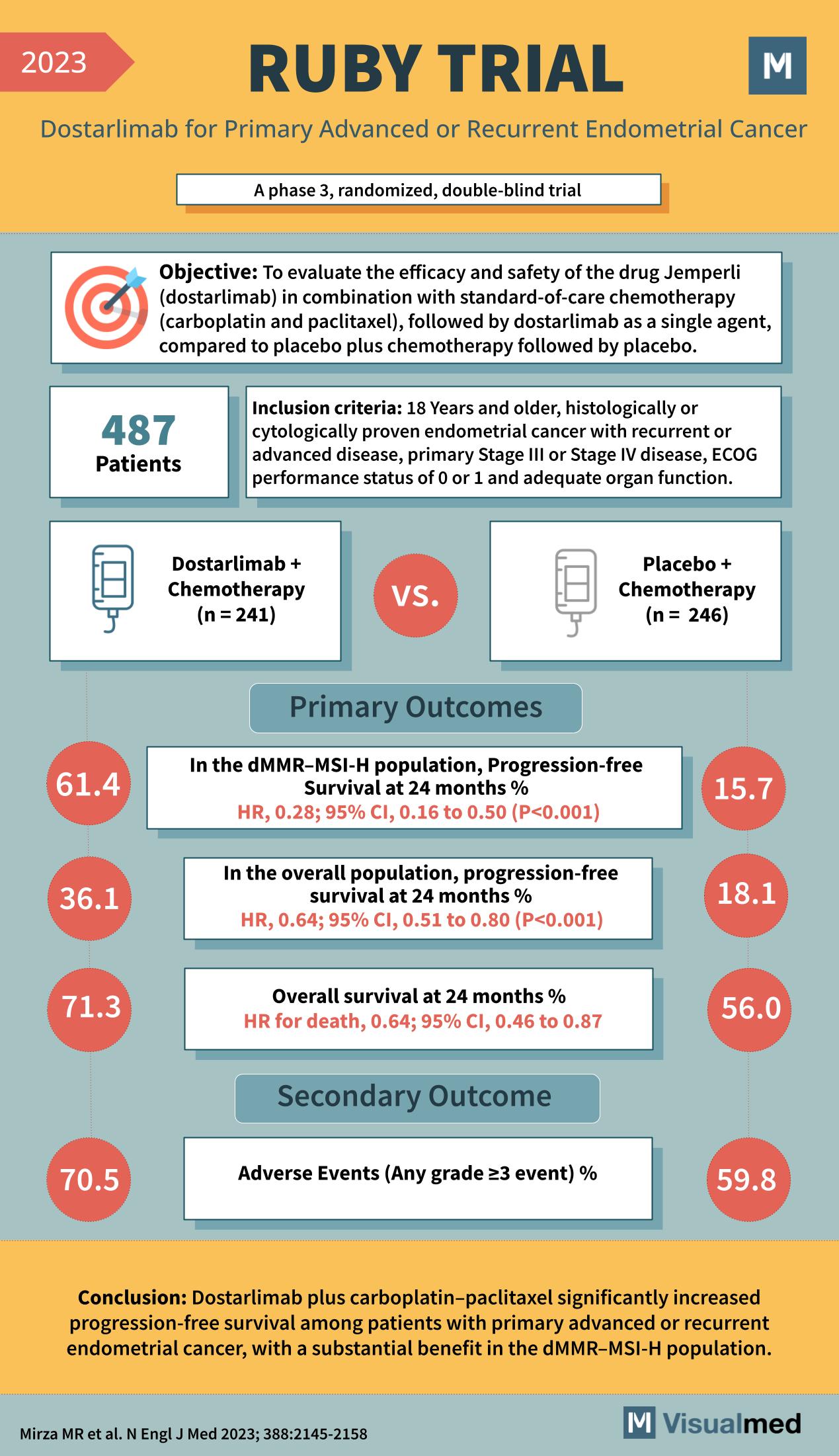
Objective of the RUBY Trial
The primary objective of the RUBY Trial was to assess the impact of Jemperli (dostarlimab) combined with a chemotherapy regimen of carboplatin and paclitaxel, followed by dostarlimab as a single agent, in comparison to placebo plus chemotherapy followed by placebo.
Inclusion Criteria and Patient Demographics
The trial involved 487 patients who met the inclusion criteria: individuals 18 years or older with histologically or cytologically proven endometrial cancer that was recurrent or at an advanced stage, specifically stage III or IV. Participants also had to have an ECOG performance status of 0 or 1 and adequate organ function.
Treatment Groups and Patient Allocation
Patients were allocated to two treatment groups: 241 patients received dostarlimab plus chemotherapy, while 246 were given a placebo in addition to chemotherapy.
Key Findings from the RUBY Trial
Primary Outcomes
The RUBY Trial reported its primary outcomes with a focus on progression-free survival at 24 months. In the dMMR-MSI-H population, the progression-free survival was a notable 61.4% with dostarlimab, compared to 15.7% with the placebo. The overall population showed a progression-free survival of 36.1% with dostarlimab against 18.1% with the placebo. Furthermore, overall survival at 24 months was 71.3% in the treatment group versus 56.0% in the placebo group.
Secondary Outcome
Adverse events of any grade ≥3 were observed in 70.5% of patients receiving the treatment compared to 59.8% in the placebo group, which is an important consideration for patient management.
Conclusion
The RUBY Trial concluded that the addition of dostarlimab to the standard chemotherapy regimen significantly increased progression-free survival among patients with primary advanced or recurrent endometrial cancer, with the dMMR-MSI-H population deriving substantial benefit.
Implications for Future Treatment
These compelling results from the RUBY Trial suggest that incorporating dostarlimab into the treatment protocol could redefine the standard care for endometrial cancer, particularly for patients with dMMR-MSI-H. The trial’s outcomes not only provide hope for better survival rates but also emphasize the importance of tailored treatments based on genetic profiles.