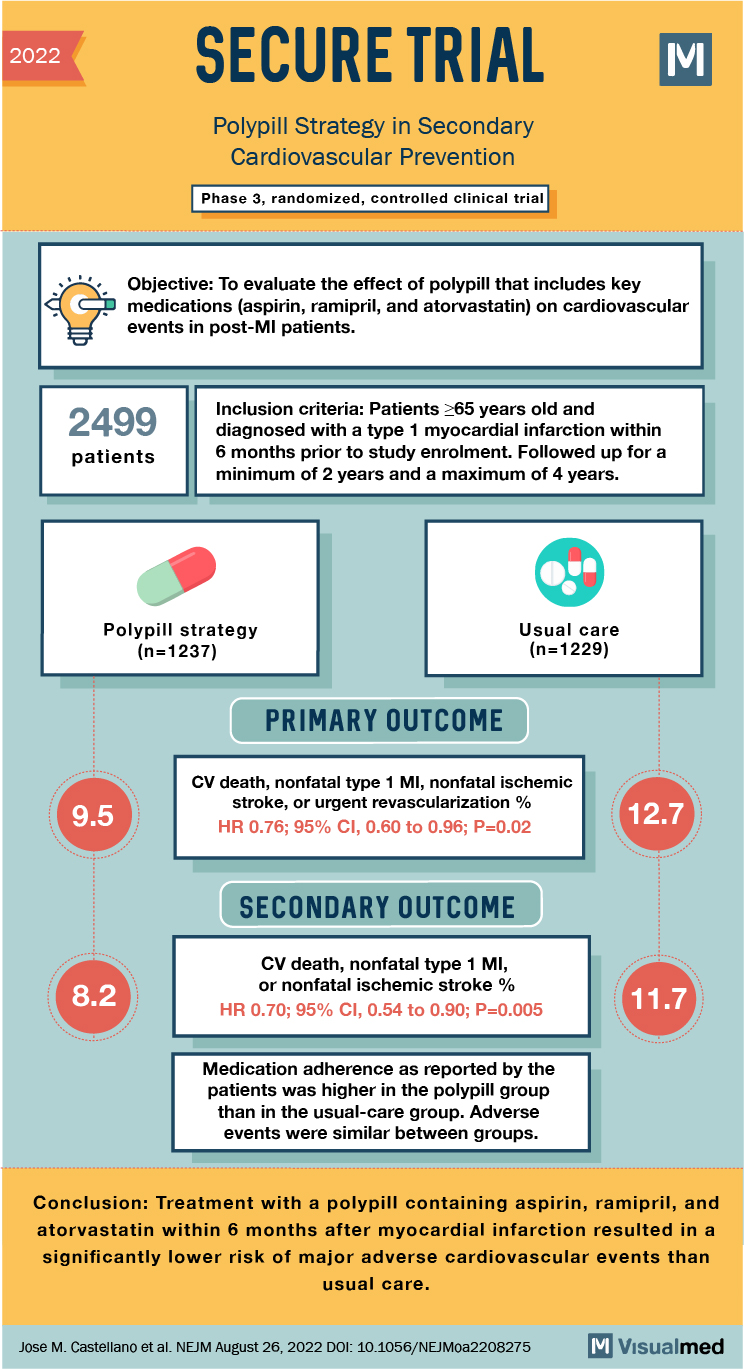
2022 SECURE TRIAL Polypill Strategy in Secondary Cardiovascular Prevention Phase 3, randomized, controlled clinical trial Objective: To evaluate the effect of polypill that includes key medications (aspirin, ramipril, and atorvastatin) on cardiovascular events in post-Ml patients. 2499 Inclusion criteria: Patients >65 years old and diagnosed with a type 1 myocardial infarction within 6 months prior to study enrolment. Followed up for a minimum of 2 years and a maximum of 4 years. patients Polypill strategy (n=1237) Usual care (n=1229) PRIMARY OUTCOME 9.5 CV death, nonfatal type 1 MI, nonfatal ischemic stroke, or urgent revascularization % HR 0.76; 95% CI, 0.60 to 0.96; P=0.02 12.7 SECONDARY OUTCOME 8.2 CV death, nonfatal type 1 MI, or nonfatal ischemic stroke % HR 0.70; 95% CI, 0.54 to 0.90; P=0.005 11.7 Medication adherence as reported by the patients was higher in the polypill group than in the usual-care group. Adverse events were similar between groups. Conclusion: Treatment with a polypill containing aspirin, ramipril, and atorvastatin within 6 months after myocardial infarction resulted in a significantly lower risk of major adverse cardiovascular events than usual care. Jose M. Castellano et al. NEJM August 26, 2022 DOI: 10.1056/NEJMoa2208275