Semaglutide Improve CV events? SELECT Trial Summary
The SELECT trial, a pivotal study published in the New England Journal of Medicine (NEJM) in November 2023, has offered the medical community valuable insights into the treatment of cardiovascular (CV) events in patients with obesity but without diabetes. The rigorously designed SELECT trial stands as a milestone in cardiovascular research, especially pertinent for individuals with preexisting cardiovascular diseases and overweight or obesity issues.
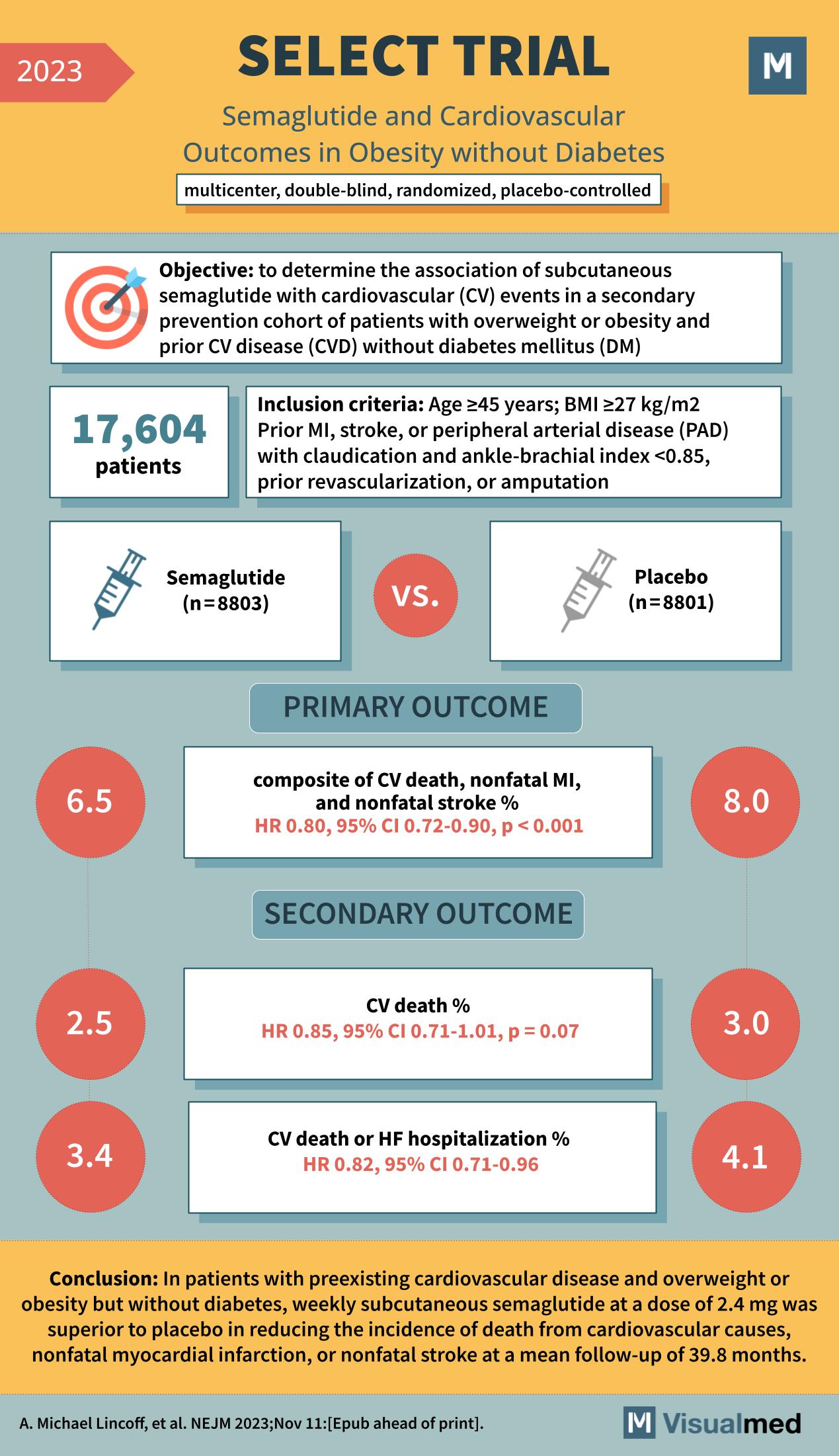
The SELECT trial was a multicenter, double-blind, randomized, placebo-controlled study that sought to determine the association of subcutaneous semaglutide with cardiovascular events. The trial involved 17,604 patients who were randomized to receive either semaglutide or a placebo. The objective was clear: to explore the impact of semaglutide on patients with a BMI of ≥27 kg/m2, aged ≥45 years, who had prior instances of myocardial infarction (MI), stroke, or peripheral arterial disease (PAD) with specific conditions, but who did not have diabetes mellitus (DM).
The primary outcome of the SELECT trial was a composite of cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke. The results were significant, with the semaglutide group showing a 6.5% incidence compared to 8.0% in the placebo group, indicating a hazard ratio (HR) of 0.80 and a 95% confidence interval (CI) ranging from 0.72 to 0.90. This marked a statistically significant finding with a p-value of <0.001, underscoring semaglutide’s efficacy in reducing major cardiovascular events.
The study also investigated secondary outcomes, including the individual components of the primary composite outcome. The incidence of CV death alone was 2.5% in the semaglutide group versus 3.0% in the placebo group, resulting in an HR of 0.85, although this did not reach statistical significance, with a p-value of 0.07. Moreover, the rate of CV death or heart failure hospitalization was 3.4% for semaglutide against 4.1% for placebo, which was statistically significant with an HR of 0.82 and a p-value of <0.96.
The conclusion drawn from the SELECT trial is robust and promising: weekly subcutaneous semaglutide at a dose of 2.4 mg was superior to placebo in reducing the incidence of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke over an average follow-up period of 39.8 months. This conclusion has profound implications for the management of cardiovascular risk in patients with obesity, offering a new therapeutic avenue to consider.
The SELECT trial is not just a clinical study; it is a testament to the evolving landscape of cardiovascular risk management. It highlights the importance of innovative approaches to address the complex interplay between obesity and cardiovascular health. The trial’s findings are particularly crucial for cardiologists and primary care physicians who are at the frontline of managing patients with cardiovascular risk factors.
Moreover, the SELECT trial underscores the need for tailored treatments for patients with specific profiles — in this case, those with obesity but without diabetes. The study provides a data-driven basis for healthcare professionals to make informed decisions about incorporating semaglutide into their clinical practice.
As we continue to disseminate the findings from the SELECT trial, it is essential to appreciate the trial’s contribution to evidence-based medicine. It serves as a guiding light for future research and paves the way for more targeted, effective treatments for cardiovascular disease in special populations. The SELECT trial is a leap forward in our quest to reduce the burden of cardiovascular disease, and its findings will resonate for years to come in the medical community.