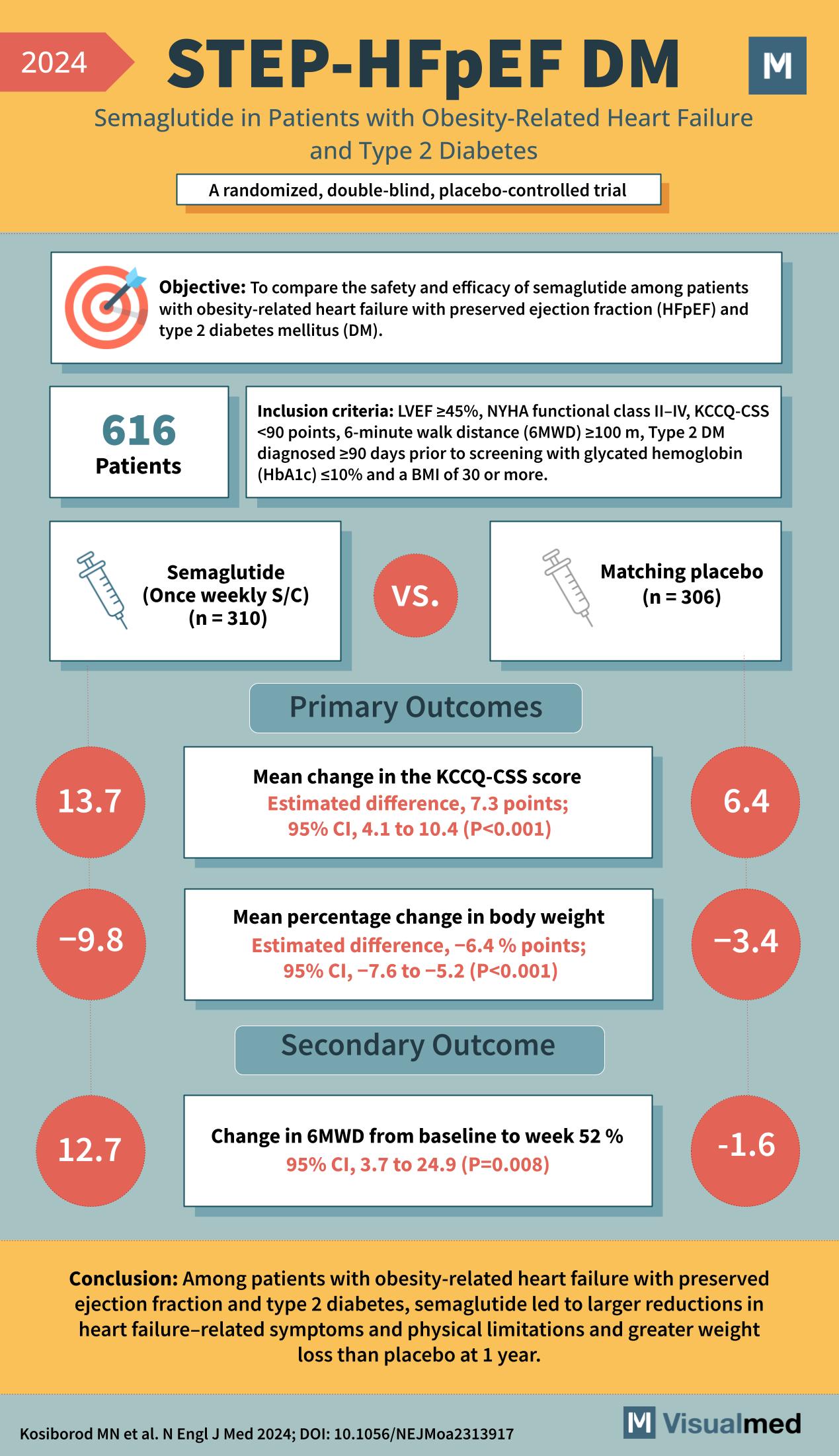
- Study Name: STEP-HFpEF DM
- Medication: Semaglutide in Patients with Obesity-Related Heart Failure and Type 2 Diabetes
- Type of Study: Randomized, double-blind, placebo-controlled trial
- Objective: To compare the safety and efficacy of semaglutide among patients with obesity-related heart failure with preserved ejection fraction (HFpEF) and type 2 diabetes mellitus (DM).
- Inclusion Criteria:
- Left Ventricular Ejection Fraction (LVEF) >45%
- New York Heart Association (NYHA) functional class II-IV
- Kansas City Cardiomyopathy Questionnaire – Clinical Summary Score (KCCQ-CSS) <90 points
- 6-minute walk distance (6MWD) ≥100 m
- Type 2 DM diagnosed ≥90 days prior to screening
- Glycated hemoglobin (HbA1c) ≤10%
- Body Mass Index (BMI) of 30 or more
- Number of Patients: 616
- Semaglutide (once weekly S/C): 310
- Matching placebo: 306
- Primary Outcomes:
- Mean change in the KCCQ-CSS score: 13.7 for Semaglutide group vs. 6.4 for placebo
- Estimated difference: 7.3 points
- 95% Confidence Interval (CI): 4.1 to 10.4 (P<0.001)
- Mean percentage change in body weight: -9.8% for Semaglutide group vs. -3.4% for placebo
- Estimated difference: -6.4 percentage points
- 95% CI: -7.6 to -5.2 (P<0.001)
- Mean change in the KCCQ-CSS score: 13.7 for Semaglutide group vs. 6.4 for placebo
- Secondary Outcome:
- Change in 6MWD from baseline to week 52 %: 12.7 for Semaglutide group vs. -1.6 for placebo
- 95% CI: 3.7 to 24.9 (P=0.008)
- Change in 6MWD from baseline to week 52 %: 12.7 for Semaglutide group vs. -1.6 for placebo
- Conclusion: Semaglutide led to larger reductions in heart failure-related symptoms and physical limitations and greater weight loss than placebo at 1 year.
- Reference: Kosiborod MN et al. N Engl J Med 2024; DOI: 10.1056/NEJMoa2313917
The study suggests that Semaglutide is effective in reducing symptoms and weight in patients with obesity-related heart failure with preserved ejection fraction and type 2 diabetes when compared to a placebo