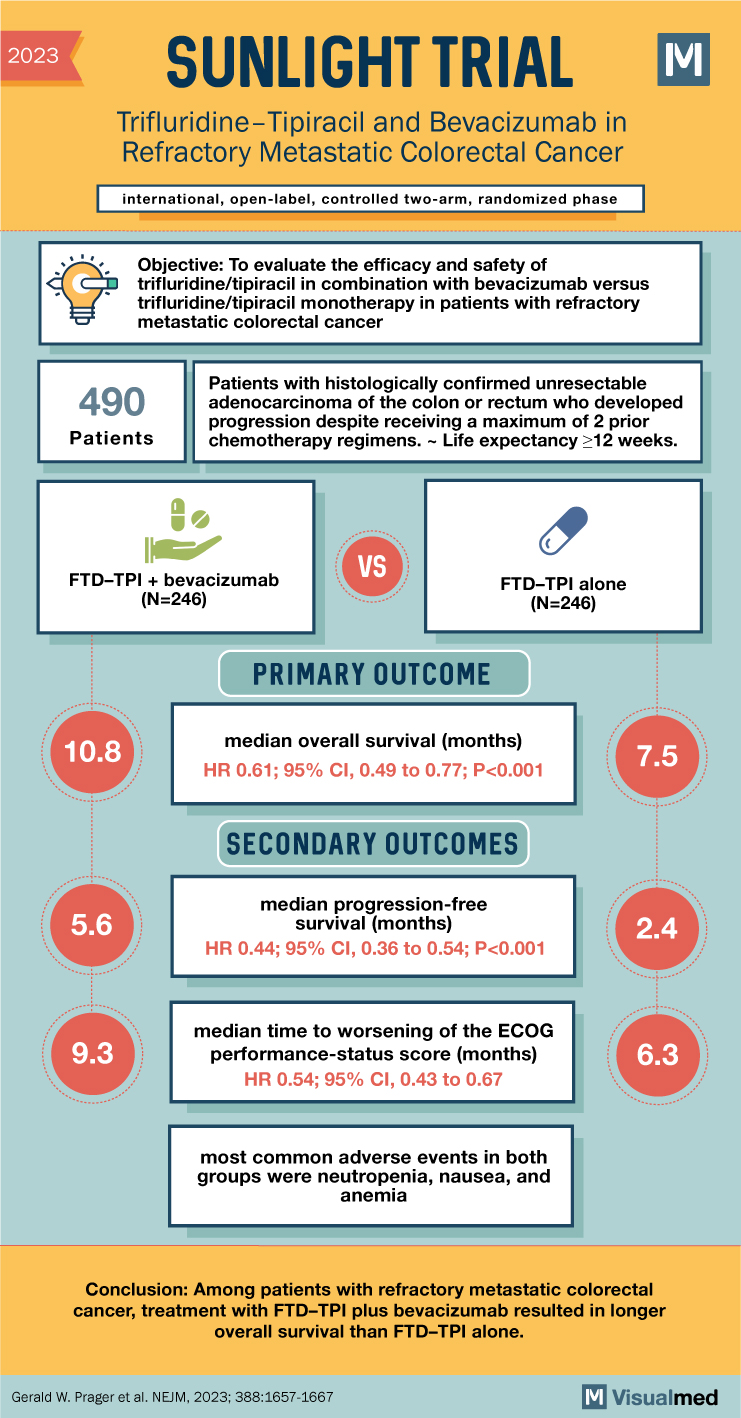
The groundbreaking “SUNLIGHTTrial” recently highlighted the potential of the combined treatment of Trifluridine–Tipiracil (FTD–TPI) and Bevacizumab in extending the lifespan of patients with advanced colorectal cancer. The promising phase 3 study builds on prior research suggesting the efficacy of FTD–TPI in these cases, and points to the enhanced potential of FTD–TPI when used in combination with Bevacizumab.
In this trial, 492 adult patients were evenly assigned into two distinct groups. Each patient had received no more than two previous chemotherapy regimens for advanced colorectal cancer. One group received the combination of FTD–TPI and Bevacizumab, while the other was treated with FTD–TPI alone. The trial aimed to analyze overall survival, progression-free survival, and safety outcomes, including the time to the worsening of patients’ Eastern Cooperative Oncology Group (ECOG) performance-status score.
The combination treatment group reported a median overall survival of 10.8 months, a significant improvement compared to the 7.5 months observed in the group treated with FTD–TPI alone. This corresponds to a hazard ratio for death of 0.61, demonstrating a 39% reduction in the risk of death for patients in the combination group (95% confidence interval [CI], 0.49 to 0.77; P<0.001).
Furthermore, combination therapy also showed a remarkable improvement in progression-free survival. Patients in the combination group experienced a median progression-free survival of 5.6 months, more than double the 2.4 months noted in the FTD–TPI group. The hazard ratio for disease progression or death was 0.44, indicating a reduction of the risk by 56% (95% CI, 0.36 to 0.54; P<0.001).
The trial’s safety evaluations revealed that the most common adverse events for both groups were neutropenia, nausea, and anemia. Importantly, no treatment-related deaths were reported. Additionally, the combined therapy also showed a slower time to the worsening of the ECOG performance-status score. The median time to the increase of the ECOG score from 0 or 1 to 2 or higher was 9.3 months in the combination group and 6.3 months in the FTD–TPI group, a reduction in risk of 46% (hazard ratio, 0.54; 95% CI, 0.43 to 0.67).
In conclusion, the Sunlight Trial has shown promising results for patients with refractory metastatic colorectal cancer. The combined treatment of FTD–TPI and Bevacizumab led to significantly longer overall survival and progression-free survival times compared to FTD–TPI alone. Further studies will be needed to confirm these findings and to refine the application of these treatments. Nonetheless, the findings represent a significant stride forward in the battle against advanced colorectal cancer.