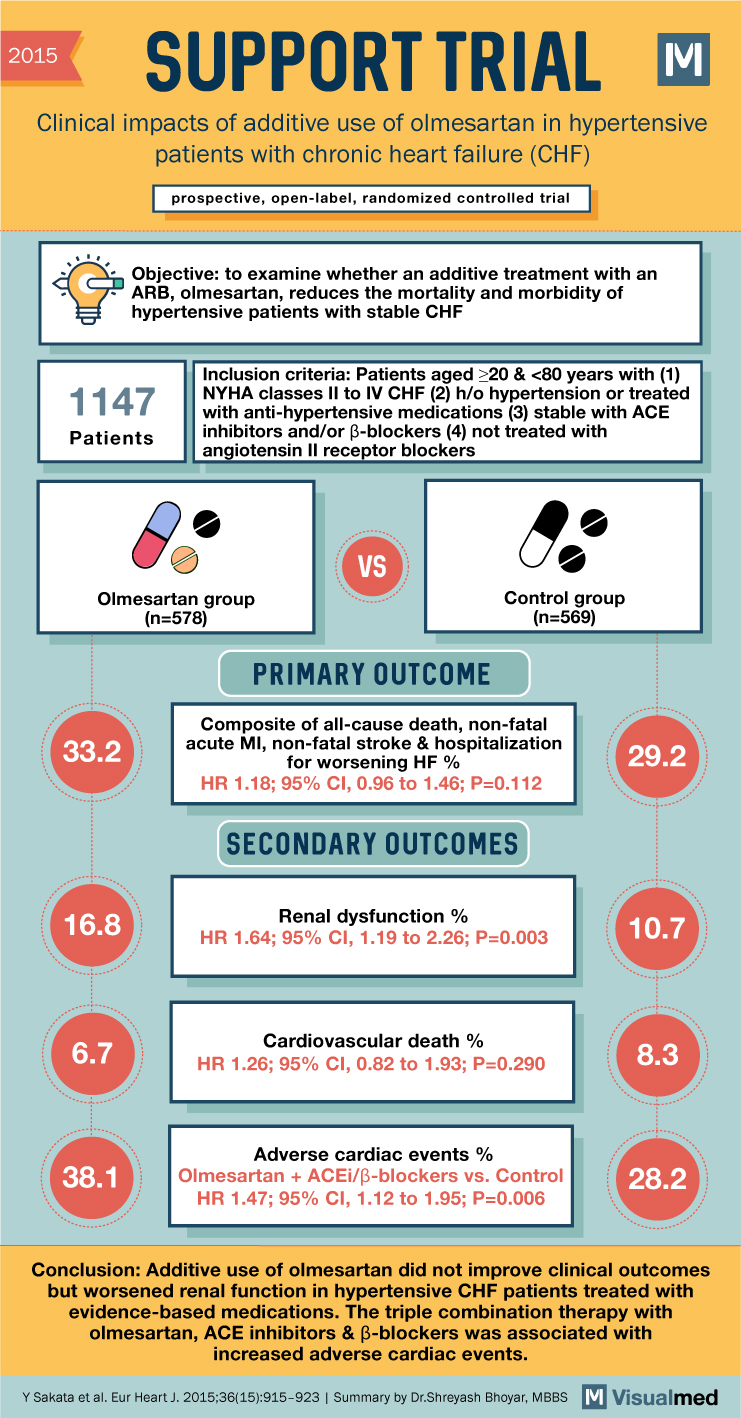
2015 SUPPORT TRIAL M Clinical impacts of additive use of olmesartan in hypertensive patients with chronic heart failure (CHF) prospective, open-label, randomized controlled trial Objective: to examine whether an additive treatment with an ARB, olmesartan, reduces the mortality and morbidity of hypertensive patients with stable CHF 1147 Patients Inclusion criteria: Patients aged ≥20 & <80 years with (1) NYHA classes II to IV CHF (2) h/o hypertension or treated with anti-hypertensive medications (3) stable with ACE inhibitors and/or B-blockers (4) not treated with angiotensin II receptor blockers VS Olmesartan group (n=578) Control group (n=569) 33.2 PRIMARY OUTCOME Composite of all-cause death, non-fatal acute MI, non-fatal stroke & hospitalization for worsening HF % HR 1.18; 95% CI, 0.96 to 1.46; P=0.112 SECONDARY OUTCOMES Renal dysfunction % 29.2 16.8 HR 1.64; 95% CI, 1.19 to 2.26; P=0.003 10.7 Cardiovascular death % 6.7 8.3 HR 1.26; 95% CI, 0.82 to 1.93; P=0.290 Adverse cardiac events % 38.1 Olmesartan + ACEi/ẞ-blockers vs. Control HR 1.47; 95% CI, 1.12 to 1.95; P=0.006 28.2 Conclusion: Additive use of olmesartan did not improve clinical outcomes but worsened renal function in hypertensive CHF patients treated with evidence-based medications. The triple combination therapy with olmesartan, ACE inhibitors & ẞ-blockers was associated with increased adverse cardiac events. Y Sakata et al. Eur Heart J. 2015;36(15):915-923