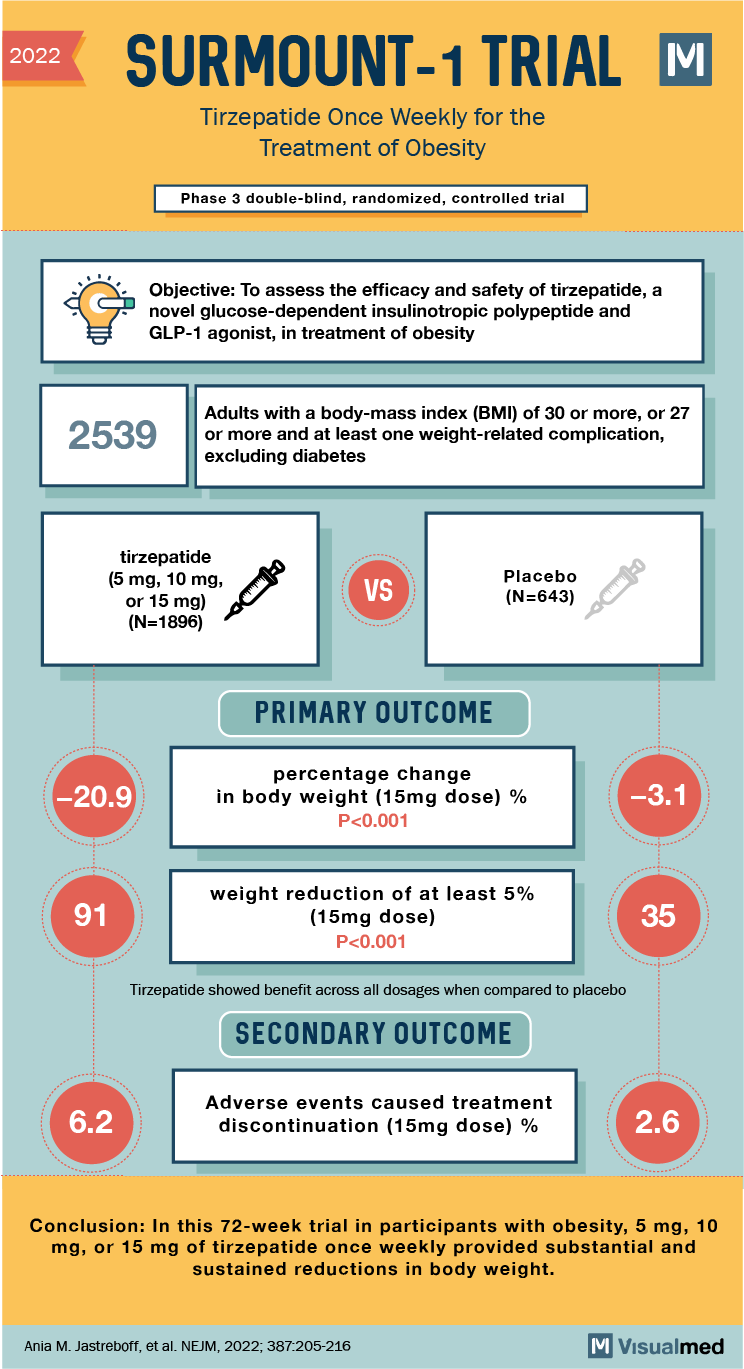
2022 SURMOUNT-1 TRIAL M Tirze patide Once Weekly for the Treatment of Obesity Phase 3 double-blind, randomized, controlled trial Objective: To assess the efficacy and safety of tirzepatide, a novel glucose-dependent insulinotropic polypeptide and GLP-1 agonist, in treatment of obesity 2539 Adults with a body-mass index (BMI) of 30 or more, or 27 or more and at least one weight-related complication, excluding diabetes tirzepatide (5 mg, 10 mg, or 15 mg) (N=1896) VS Placebo (N=643) (PRIMARY OUTCOME -20.9 percentage change in body weight (15mg dose) % P<0.001 -3.1 91 weight reduction of at least 5% (15mg dose) P<0.001 Tirzepatide showed benefit across all dosages when compared to placebo SECONDARY OUTCOME 6.2 Adverse events caused treatment discontinuation (15mg dose) % 2.6 Conclusion: In this 72-week trial in participants with obesity, 5 mg, 10 mg, or 15 mg of tirzepatide once weekly provided substantial and sustained reductions in body weight. Ania M. Jastreboff, et al. NEJM, 2022; 387:205-216