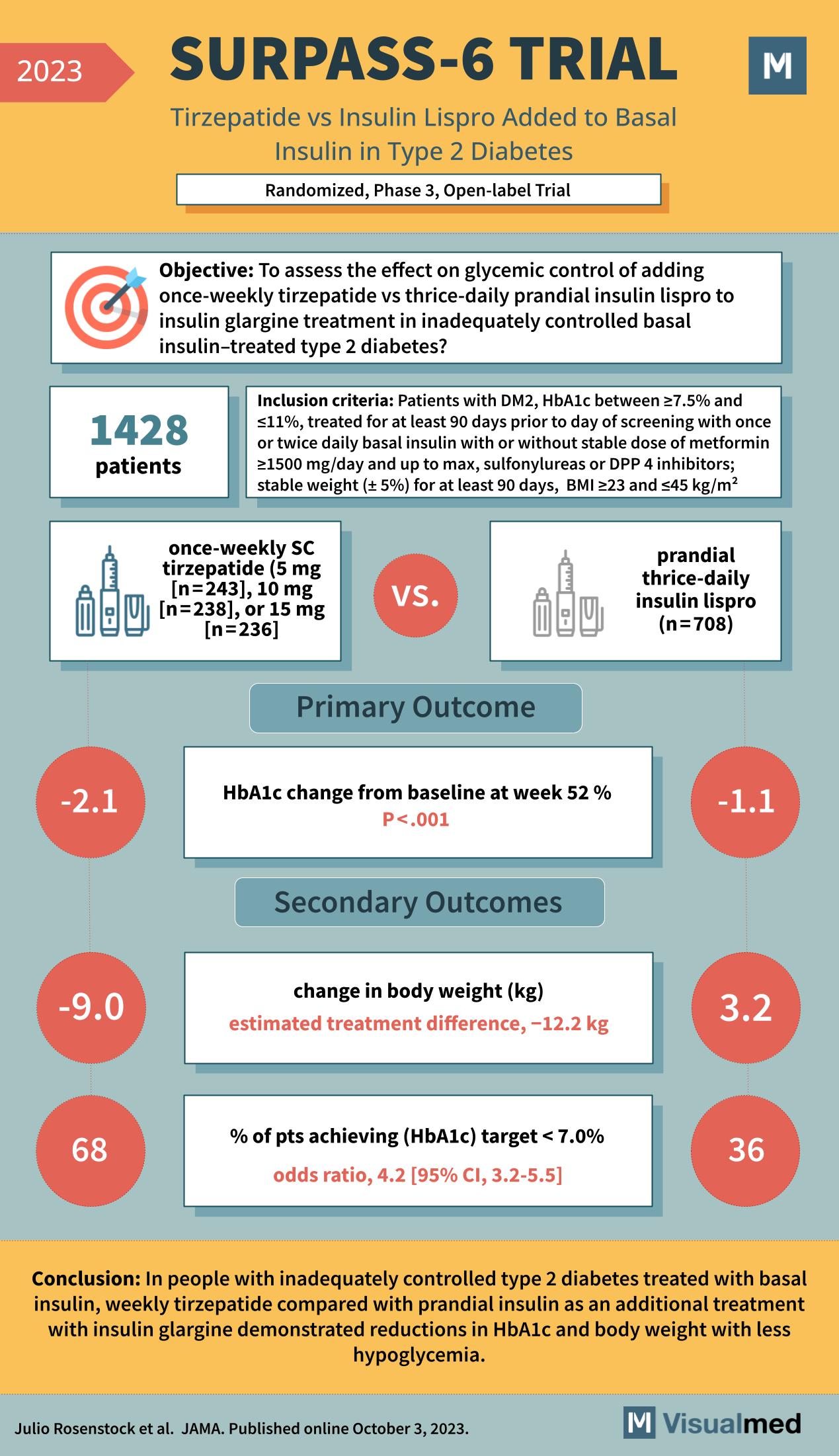
The SURPASS-6 trial, featured in JAMA in October 2023, represents a significant advancement in the treatment of type 2 diabetes. This randomized, phase 3, open-label trial was designed to evaluate the effects of once-weekly subcutaneous (SC) tirzepatide versus thrice-daily prandial insulin lispro when added to basal insulin glargine in patients with inadequately controlled type 2 diabetes.
With 1428 patients enrolled, the trial set rigorous inclusion criteria, selecting participants with type 2 diabetes (DM2), HbA1c levels between 7.5% and 11%, and who had been on a stable regimen of diabetes medications, including metformin and either sulfonylureas or DPP-4 inhibitors. The study also required patients to have maintained a stable weight and body mass index (BMI) within specific parameters for at least 90 days prior to the study.
The SURPASS-6 trial’s primary outcome was the change in HbA1c levels from baseline at week 52. The results were striking: those in the tirzepatide group experienced a significant reduction in HbA1c of 2.1%, compared to a reduction of 1.1% in the insulin lispro group, with the difference being statistically significant (p<0.001).
Secondary outcomes of the trial included changes in body weight and the percentage of patients achieving an HbA1c target of less than 7.0%. Patients receiving tirzepatide saw a notable decrease in body weight, averaging a loss of 9.0 kg, compared to a 3.2 kg increase in the insulin lispro group. This estimated treatment difference of -12.2 kg strongly favored tirzepatide. Moreover, a significant 68% of patients in the tirzepatide group achieved the target HbA1c, compared to 36% in the insulin lispro group, with an odds ratio of 4.2 (95% CI, 3.2-5.5).
The conclusion of the SURPASS-6 trial is a beacon of hope for those struggling with type 2 diabetes management. In patients with inadequately controlled type 2 diabetes on basal insulin, weekly tirzepatide proved superior to prandial insulin lispro as an adjunct treatment with insulin glargine, demonstrating not just reductions in HbA1c but also significant weight loss with a lower incidence of hypoglycemia.
The trial’s outcomes suggest that tirzepatide may be a more effective option for patients who have not achieved their glycemic goals with existing insulin therapies. The SURPASS-6 trial adds considerable weight to the evidence supporting the use of GLP-1 receptor agonists like tirzepatide in the management of type 2 diabetes, particularly for those patients who also need to manage their weight.
The SURPASS-6 trial’s findings have the potential to influence clinical guidelines and reshape the therapeutic approach for type 2 diabetes, emphasizing the importance of individualized patient care and the need for innovative treatments to manage this chronic condition effectively.