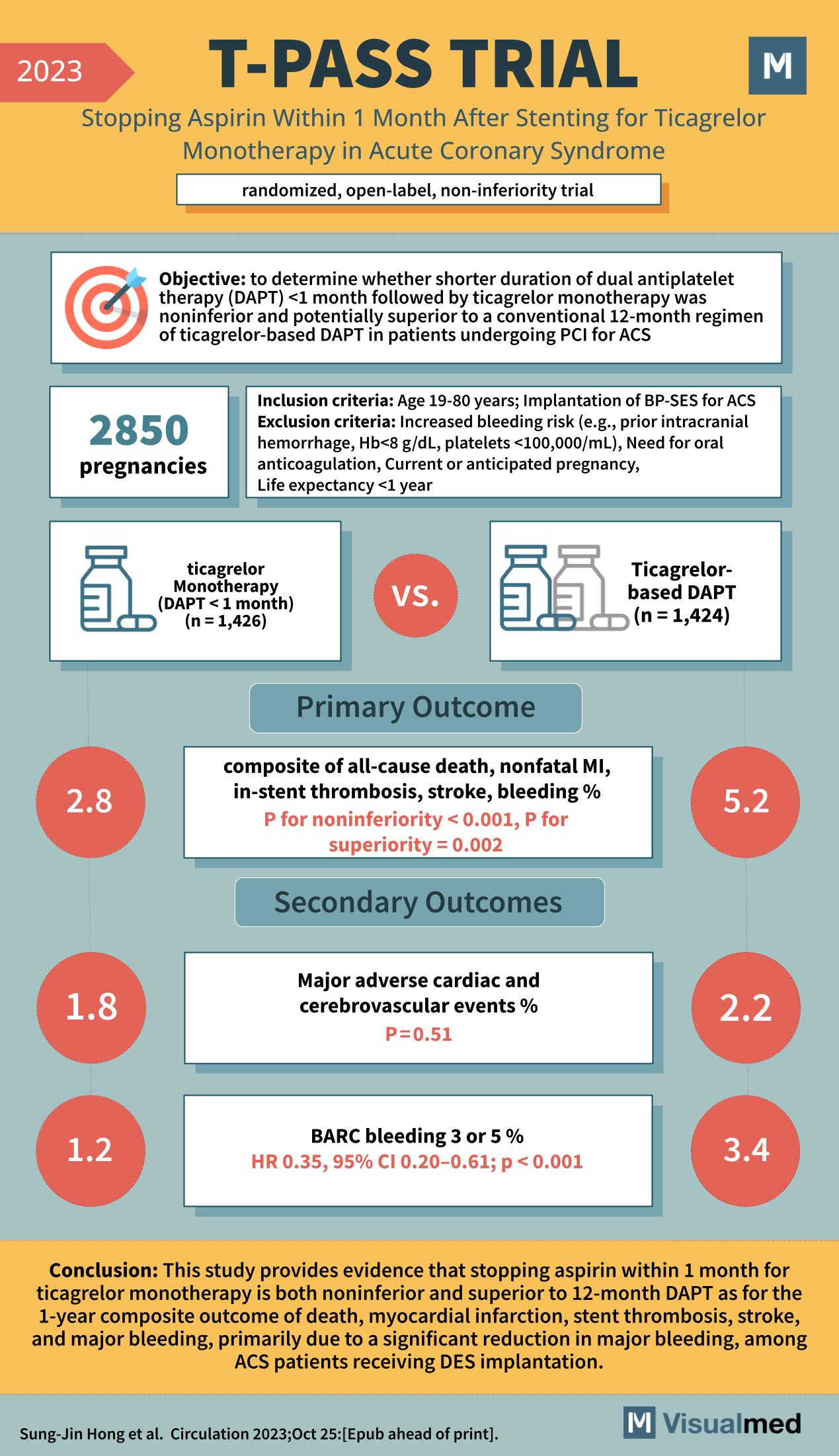
T-PASS Trial Summary
The management of Acute Coronary Syndrome (ACS) after stenting has been a hot topic for medical researchers for years. The recent T-PASS Trial in 2023 has shed light on the possible advantages of shifting from the conventional 12-month dual antiplatelet therapy (DAPT) to a shorter one-month duration followed by ticagrelor monotherapy.
Objective of the T-PASS Trial
The primary aim of this open-label, non-inferiority trial was to ascertain if a shorter duration of DAPT (less than a month), succeeded by ticagrelor monotherapy, could match or even surpass the results of the traditional 12-month ticagrelor-based DAPT in patients undergoing Percutaneous Coronary Intervention (PCI) for ACS.
Inclusion and Exclusion Criteria
Patients aged between 19 to 80 years, who underwent implantation of BP-SES for ACS, were considered for the study. However, those at a heightened risk for bleeding (e.g., prior intracranial hemorrhage, low platelet count, or high Hemoglobin levels) and other specific conditions such as the need for oral anticoagulation, current or anticipated pregnancy, or a life expectancy of less than a year were excluded.
Key Findings
The trial divided patients into two groups:
- Ticagrelor Monotherapy Group (DAPT for less than a month): 1,426 participants
- Ticagrelor-based DAPT Group: 1,424 participants
The primary outcome centered on a composite of all-cause death, nonfatal MI, in-stent thrombosis, stroke, and bleeding. Remarkably, the ticagrelor monotherapy group exhibited superior results with a percentage of 2.8% as compared to the 5.2% in the DAPT group. The statistical significance was evident with a P-value for noninferiority less than 0.001 and for superiority being 0.002. Furthermore, the secondary outcomes were assessed, focusing on major adverse cardiac and cerebrovascular events and BARC bleeding 3 or 5. The results were as follows:
- Major adverse events were relatively equal in both groups, with 1.8% in the monotherapy group and 2.2% in the DAPT group, producing a P-value of 0.51.
- Notably, the BARC bleeding 3 or 5 was significantly lower in the monotherapy group, standing at 1.2%, compared to 3.4% in the DAPT group, with a hazard ratio of 0.35 (95% CI 0.20-0.61) and a P-value less than 0.001.
Conclusion
The T-PASS trial provides compelling evidence suggesting that for ACS patients receiving DES implantation, ceasing aspirin within a month for ticagrelor monotherapy might not only be noninferior but also superior to the 12-month DAPT. This was chiefly seen in the dramatic reduction in major bleeding events, a critical factor in post-stenting management. The study, indeed, offers promising insights for ACS management post-stenting, paving the way for potentially safer and more effective treatment regimens in the future.